A) oxidizing action of \[{{H}_{2}}{{O}_{2}}\]
B) reducing action of \[{{H}_{2}}{{O}_{2}}\]
C) alkaline nature of \[{{H}_{2}}{{O}_{2}}\]
D) acidic nature of \[{{H}_{2}}{{O}_{2}}\]
Correct Answer: B
Solution :
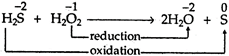 Thus, \[{{H}_{2}}{{O}_{2}}\] is acting as an oxidizing agent here.
Thus, \[{{H}_{2}}{{O}_{2}}\] is acting as an oxidizing agent here.
You need to login to perform this action.
You will be redirected in
3 sec
