A) +10
B) +5
C) +6
D) +3
Correct Answer: D
Solution :
\[Cr{{O}_{5}}\]has the following structure: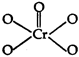 Hence, oxidation state of chromium can be calculated as: \[x+4\,(-1)+1\,(-\,2)=0\] (peroxide-o-atoms) or \[x-4-2=0\] \[x=+\,6\]
Hence, oxidation state of chromium can be calculated as: \[x+4\,(-1)+1\,(-\,2)=0\] (peroxide-o-atoms) or \[x-4-2=0\] \[x=+\,6\]
You need to login to perform this action.
You will be redirected in
3 sec
