A) Tetrahedral
B) Pyramidal
C) Angular
D) Planar
Correct Answer: C
Solution :
The electron dot-structure of \[{{H}_{2}}O\] is \[\underset{(lps\,+\,\sigma \,-\,bps\,=\,2\,+\,2\,=\,4)}{\mathop{H--\underset{.\,\,.}{\overset{.\,\,.}{\mathop{O}}}\,--H}}\,\] Hence, oxygen atom undergoes \[s{{p}^{3}}\text{-}\]hybridisation. It should be tetrahedral, but due to presence of 2 lone-pairs of electron, it is angular with H?O?H, bond angle\[=104.5{}^\circ .\]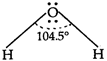
You need to login to perform this action.
You will be redirected in
3 sec
