A) \[C{{H}_{3}}--\underset{C{{H}_{3}}}{\overset{+}{\mathop{\underset{|}{\mathop{C}}\,}}}\,--H\]
B) \[C{{H}_{3}}--\underset{C{{H}_{3}}}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{\overset{|}{\mathop{{{C}^{\,+}}}}\,}}\,}}}\,\]
C) \[\overset{+}{\mathop{C{{H}_{3}}}}\,\]
D) \[C{{H}_{3}}\overset{+}{\mathop{C{{H}_{2}}}}\,\]
Correct Answer: B
Solution :
The most stable carbocation is r-alkyl carbocation because the order of stability of alkyl carbocation is t-alkyl > s-alkyl > p-alkyl >\[CH_{3}^{+}\] carbocation. This stability order is described with the help of hyper conjugation and inductive effect. On the basis of hyper conjugation, \[{{(C{{H}_{3}})}_{2}}\overset{+}{\mathop{CH}}\,\] shows six resonating structures due to presence of six \[\alpha \,C--H\] bonds,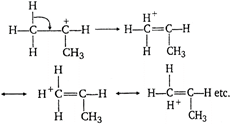 \[C{{H}_{3}}-\underset{C{{H}_{3}}}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{\,\,\,{{C}^{+}}}}}\,}}}\,\]shows nine resonating structures due to presence of nine a C? H bonds. \[\overset{+}{\mathop{C{{H}_{3}}}}\,\]does not show the property of resonance while \[C{{H}_{3}}--\overset{+}{\mathop{C{{H}_{2}}}}\,\]shows three resonating structures due to presence of three \[\alpha \,C--H\] bonds. Hence, larger number of resonating structures are possible in (2), so it is most stable. The above order of stability is also explained with the help of (+) I-effect of\[-C{{H}_{3}}\] group. More the number of \[-C{{H}_{3}}\] group more will be tendency to displace the electrons towards positive charged carbon of carbocation. Thus (+) charge is decreased or compensated i.e., positive charge is decreased and stability of carbocation is increased.
\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{\,\,\,{{C}^{+}}}}}\,}}}\,\]shows nine resonating structures due to presence of nine a C? H bonds. \[\overset{+}{\mathop{C{{H}_{3}}}}\,\]does not show the property of resonance while \[C{{H}_{3}}--\overset{+}{\mathop{C{{H}_{2}}}}\,\]shows three resonating structures due to presence of three \[\alpha \,C--H\] bonds. Hence, larger number of resonating structures are possible in (2), so it is most stable. The above order of stability is also explained with the help of (+) I-effect of\[-C{{H}_{3}}\] group. More the number of \[-C{{H}_{3}}\] group more will be tendency to displace the electrons towards positive charged carbon of carbocation. Thus (+) charge is decreased or compensated i.e., positive charge is decreased and stability of carbocation is increased.
You need to login to perform this action.
You will be redirected in
3 sec
