A) \[4a=\sqrt{3}\,r\]
B) \[4r=\sqrt{3}\,a\]
C) \[4r=\sqrt{2}\,a\]
D) \[4r=\frac{a}{\sqrt{2}}\]
Correct Answer: C
Solution :
Given, the edge length of the unit cell = a and, radius of the sphere = r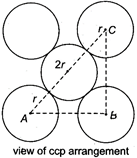 Consider \[\Delta \,ABC,\] \[A{{C}^{2}}=A{{B}^{2}}+A{{C}^{2}}\] \[={{a}^{2}}+{{a}^{2}}\] \[=2{{a}^{2}}\] \[\therefore \] \[AC=\sqrt{2}\cdot a\] But, from the figure, AC = 4r \[\therefore \] \[4r=\sqrt{2}\cdot a\]
Consider \[\Delta \,ABC,\] \[A{{C}^{2}}=A{{B}^{2}}+A{{C}^{2}}\] \[={{a}^{2}}+{{a}^{2}}\] \[=2{{a}^{2}}\] \[\therefore \] \[AC=\sqrt{2}\cdot a\] But, from the figure, AC = 4r \[\therefore \] \[4r=\sqrt{2}\cdot a\]
You need to login to perform this action.
You will be redirected in
3 sec
