-
question_answer1) The catenation tendency of C, Si and Ge is in the order Ge < Si < C. The bond energies \[\left( \operatorname{in}\operatorname{K}\operatorname{J}\operatorname{mo}{{1}^{-1}} \right)\]of C-C, Si-Si and Ge-Ge bonds are respectively:
JEE Main Online Paper ( Held On 25 April 2013 )
A)
348, 297, 260
done
clear
B)
297, 348, 260
done
clear
C)
348, 260, 297
done
clear
D)
260, 297, 348
done
clear
View Answer play_arrow
-
question_answer2) In which of the following exothermic reactions, the heat liberated per mole is the highest?
JEE Main Online Paper ( Held On 25 April 2013 )
A)
\[CaO+{{H}_{2}}O\to Ca{{(OH)}_{2}}\]
done
clear
B)
\[\operatorname{SrO}+{{\operatorname{H}}_{2}}\operatorname{O}\to \operatorname{Sr}{{(\operatorname{OH})}_{2}}\]
done
clear
C)
\[\operatorname{Ba}\operatorname{O}+{{\operatorname{H}}_{2}}\operatorname{O}\to \operatorname{B}a{{(\operatorname{OH})}_{2}}\]
done
clear
D)
\[\operatorname{M}g\operatorname{O}+{{\operatorname{H}}_{2}}\operatorname{O}\to \operatorname{Mg}{{(\operatorname{OH})}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer3) Give
(A) \[n=5,\,\,{{m}_{l}}=+1\]
(B) \[n=2,\,\,l+1,\,\,\,{{m}_{l}}=-1\],\[{{\operatorname{m}}_{s}}=-1/2\]
JEE Main Online Paper ( Held On 25 April 2013 )
A)
25 and 1
done
clear
B)
8 and 1
done
clear
C)
2 and 4
done
clear
D)
4 and 1
done
clear
View Answer play_arrow
-
question_answer4) Which one of the following cannot function as an oxidizing agent?
JEE Main Online Paper ( Held On 25 April 2013 )
A)
\[{{I}^{-}}\]
done
clear
B)
\[{{S}_{(s)}}\]
done
clear
C)
\[NO_{3}^{-}(aq)\]
done
clear
D)
\[C{{r}_{2}}O_{7}^{2-}\]
done
clear
View Answer play_arrow
-
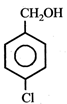
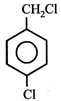
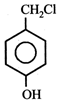
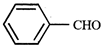
question_answer5)
Given 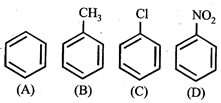 In the above compounds correct order of reactivity in electrophilic substitution reactions will be:
JEE Main Online Paper ( Held On 25 April 2013 )
In the above compounds correct order of reactivity in electrophilic substitution reactions will be:
JEE Main Online Paper ( Held On 25 April 2013 )
A)
\[b>a>c>d\]
done
clear
B)
\[d>c>b>a\]
done
clear
C)
\[a>b>c>d\]
done
clear
D)
\[b>c>a>d\]
done
clear
View Answer play_arrow
-
question_answer6) Which one of the following is the wrong assumption of kinetic theory of gases?
JEE Main Online Paper ( Held On 25 April 2013 )
A)
Momentum and energy always remain conserved
done
clear
B)
Pressure is the result of elasticcollision of molecules with the container's wall
done
clear
C)
Molecule are separated by great distances compared to their sizes.
done
clear
D)
All the molecule move in straight line between collision and with some velocity
done
clear
View Answer play_arrow
-
question_answer7) A radioactive isotope having a half?life period of 3 days received after 12 days. If 3g of the isotope is left in the container, what would be the initial mass of the isotope?
JEE Main Online Paper ( Held On 25 April 2013 )
A)
12g
done
clear
B)
36g
done
clear
C)
48g
done
clear
D)
24g
done
clear
View Answer play_arrow
-
question_answer8) Which of the following statement is not correct?
JEE Main Online Paper ( Held On 25 April 2013 )
A)
Amylopectin is branched polymer of \[\alpha \]-glucose.
done
clear
B)
Cellulose is a linear polymer of \[\beta \]glucose.
done
clear
C)
Glycogen is the food reserve of plants
done
clear
D)
All proteins are polymers of \[\alpha -\]amino acids
done
clear
View Answer play_arrow
-
question_answer9)
The major product in the following reaction 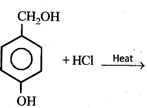 is
JEE Main Online Paper ( Held On 25 April 2013 )
is
JEE Main Online Paper ( Held On 25 April 2013 )
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer10) The ratio \[\frac{\operatorname{Kp}}{\operatorname{Kc}}\] for the reaction\[CO(g)+\frac{1}{2}{{O}_{2}}(g)\rightleftharpoons C{{O}_{2}}(g)\]is :
JEE Main Online Paper ( Held On 25 April 2013 )
A)
\[\frac{1}{\sqrt{RT}}\]
done
clear
B)
\[{{(RT)}^{1/2}}\]
done
clear
C)
RT
done
clear
D)
1
done
clear
View Answer play_arrow
-
question_answer11) Copper crystallizes in fcc with a unit length of 361 pm. What is the radius of copper atom?
JEE Main Online Paper ( Held On 25 April 2013 )
A)
157 pm
done
clear
B)
128 pm
done
clear
C)
108 pm
done
clear
D)
181 pm
done
clear
View Answer play_arrow
-
question_answer12) In which of the following sets, all the given Species are is structural?
JEE Main Online Paper ( Held On 25 April 2013 )
A)
\[{{\operatorname{CO}}_{2}},\operatorname{N}{{\operatorname{O}}_{2}},Cl{{O}_{2}}Si{{O}_{2}}\]
done
clear
B)
\[PC{{l}_{3}},AlC{{l}_{3}}BC{{l}_{3}}SbC{{l}_{3}}\]
done
clear
C)
\[{{\operatorname{BF}}_{3}},N{{F}_{3}},P{{F}_{3}},Al{{F}_{3}}\]
done
clear
D)
\[\operatorname{BF}_{4}^{-}CC{{l}_{4}},NH_{4}^{+},PCl_{4}^{+}\]
done
clear
View Answer play_arrow
-
question_answer13) Given that : (i) \[{{\Delta }_{f}}{{H}^{0}}\]of\[{{N}_{2}}O\] is \[82\operatorname{k}\operatorname{j}\]\[\operatorname{mo}{{\operatorname{l}}^{-1}}\] (ii) Bond energies of N\[\equiv \]N, N=N, O=O and N=O are 946,418,498 and 607 Kj\[{{\operatorname{mol}}^{-1}}\] respectively. The resonance energy of \[{{N}_{2}}\] O is
JEE Main Online Paper ( Held On 25 April 2013 )
A)
-88 Kj
done
clear
B)
-66 Kj
done
clear
C)
-62 Kj
done
clear
D)
-44 Kj
done
clear
View Answer play_arrow
-
question_answer14) What would be the pH of a solution obtained by mixing 5g of acetic acid and 7.5g of sodium acetate and making the volume equal to 500 mL? \[(\operatorname{Ka}=1.75\times {{10}^{-5}},\operatorname{pka}=4.6)\]
JEE Main Online Paper ( Held On 25 April 2013 )
A)
pH =4.70
done
clear
B)
pH< 4.70
done
clear
C)
pH of solution will be equal to pH of acetic acid
done
clear
D)
4.76 < pH< 5.0
done
clear
View Answer play_arrow
-
question_answer15) 6 litres of an alkene require 27 litres of oxygen at constant temperature and pressure for complete combustion. The alkene is:
JEE Main Online Paper ( Held On 25 April 2013 )
A)
Ethene
done
clear
B)
Propene
done
clear
C)
1-Butene
done
clear
D)
2-Butene
done
clear
View Answer play_arrow
-
question_answer16) Bakelite is obtained from phenol by reacting with :
JEE Main Online Paper ( Held On 25 April 2013 )
A)
Acetal
done
clear
B)
\[{{\operatorname{CH}}_{3}}\operatorname{CHO}\]
done
clear
C)
\[{{\operatorname{CH}}_{3}}\operatorname{CHO}\]
done
clear
D)
Chlorobenzene
done
clear
View Answer play_arrow
-
question_answer17) How many grams of methyl alcohol should added to 10 litre tank of water to prevent its freezing at 268 K? (\[{{\operatorname{K}}_{f}}\]for water is 1.86 K kg \[{{\operatorname{mol}}^{-1}}\])
JEE Main Online Paper ( Held On 25 April 2013 )
A)
880.07 g
done
clear
B)
899.04 g
done
clear
C)
866.02 g
done
clear
D)
868.06 g
done
clear
View Answer play_arrow
-
question_answer18) In which of the following octahedral complex species the magnitude of \[{{\Delta }_{0}}\] will be maximum?
JEE Main Online Paper ( Held On 25 April 2013 )
A)
\[{{\left[ \text{Co}\left( {{\text{H}}_{\text{2}}}{{\operatorname{O}}_{6}} \right) \right]}^{2+}}\]
done
clear
B)
\[{{\left[ \text{Co}\left( \operatorname{C}{{\operatorname{N}}_{6}} \right) \right]}^{3+}}\]
done
clear
C)
\[{{\left[ \text{Co}{{\left( {{\operatorname{C}}_{2}}{{O}_{4}} \right)}_{3}} \right]}^{3-}}\]
done
clear
D)
\[{{\left[ \text{Co}{{\left( \operatorname{N}{{\operatorname{H}}_{3}} \right)}_{6}} \right]}^{3+}}\]
done
clear
View Answer play_arrow
-
question_answer19) In nucleophilic substitution reaction, order of halogens as incoming (attacking) nucleophiles: \[{{\operatorname{I}}^{-}}>{{\operatorname{Br}}^{-}}>{{\operatorname{CI}}^{-}}\] The order of halogens as departing nucleophile should be :
JEE Main Online Paper ( Held On 25 April 2013 )
A)
\[B{{r}^{-}}>{{I}^{-}}>C{{l}^{-}}\]
done
clear
B)
\[{{I}^{-}}>B{{r}^{-}}>C{{l}^{-}}\]
done
clear
C)
\[C{{l}^{-}}>B{{r}^{-}}>{{I}^{-}}\]
done
clear
D)
\[C{{l}^{-}}>{{I}^{-}}>B{{r}^{-}}\]
done
clear
View Answer play_arrow
-
question_answer20) The Gibbs energy for the decomposition of\[A{{l}_{2}}{{O}_{3}}\]at \[{{500}^{0}}C\] is as follows: \[\frac{2}{3}A{{l}_{2}}{{O}_{3}}\to 4/3Al+{{O}_{2}},{{\Delta }_{r}}G=+940\,kJ\,mo{{l}^{-1}}\] The potential difference needed for the electrolytic reduction of aluminium oxide at\[{{500}^{0}}C\] should be atleast:
JEE Main Online Paper ( Held On 25 April 2013 )
A)
4.5 V
done
clear
B)
3.0 V
done
clear
C)
5.0 V
done
clear
D)
2.5 V
done
clear
View Answer play_arrow
-
question_answer21) Cannizaro?s reaction is not given by :
JEE Main Online Paper ( Held On 25 April 2013 )
A)
done
clear
B)
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[HCHO\]
done
clear
View Answer play_arrow
-
question_answer22) Pheol on heating with \[CHC{{l}_{3}}\]and \[\text{NaOH}\]gives Salicylaldehyde. The reaction is called:
JEE Main Online Paper ( Held On 25 April 2013 )
A)
Remer- Tiemenanraeaction
done
clear
B)
Claisen reaction
done
clear
C)
Cannizzareo?s reaction
done
clear
D)
Hell-volhard-Zelinsky reaction
done
clear
View Answer play_arrow
-
question_answer23) The inter nuclear distances in O-O bonds for \[\operatorname{O}_{2}^{+},{{\operatorname{O}}_{2}},\operatorname{O}_{2}^{-}\] and \[\operatorname{O}_{2}^{2-}\] respectively are:
A)
\[1.30\,\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.49\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.12\,\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.21\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1.49\,\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.21\,\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.12\,\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.30\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1.21\,\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.12\,\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.49\,\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.30\,\overset{\text{o}}{\mathop{\text{A}}}\,\,\]
done
clear
D)
\[1.12\,\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.21\,\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.30\,\overset{\text{o}}{\mathop{\text{A}}}\,,\,1.49\,\overset{\text{o}}{\mathop{\text{A}}}\,\,\]
done
clear
View Answer play_arrow
-
question_answer24) Among the following vitamins the one whose deficiency causes rickets (bone deficiency) is
JEE Main Online Paper ( Held On 25 April 2013 )
A)
Vitamin A
done
clear
B)
Vitamin B
done
clear
C)
Vitamin D
done
clear
D)
Vitamin C
done
clear
View Answer play_arrow
-
question_answer25) Which one of the following arrangements represent the correct order of the proton affinity of the given species:
JEE Main Online Paper ( Held On 25 April 2013 )
A)
\[{{I}^{-}}<{{F}^{-}}<H{{S}^{-}}<NH_{2}^{-}\]
done
clear
B)
\[{{\operatorname{HS}}^{-}}<\operatorname{NH}_{2}^{-}>{{\operatorname{F}}^{-}}<{{\operatorname{I}}^{-}}\]
done
clear
C)
\[{{\operatorname{F}}^{-}}<{{\operatorname{I}}^{-}}<\operatorname{NH}_{2}^{-}<{{\operatorname{HS}}^{-}}\]
done
clear
D)
\[NH_{2}^{1}<H{{S}^{-}}<{{I}^{-}}<{{F}^{-}}\]
done
clear
View Answer play_arrow
-
question_answer26) Carbylamines forms from aliphatic or aromatic primary amine via which of the following intermediates?
JEE Main Online Paper ( Held On 25 April 2013 )
A)
Carbanion
done
clear
B)
Carbine
done
clear
C)
Carbonation
done
clear
D)
Carbon radical
done
clear
View Answer play_arrow
-
question_answer27) 10 mL of 2 (M) of \[\operatorname{NaOH}\] solution is added to 200 mL of 0.5 (M) of NaOH solution. What in the final concentration?
JEE Main Online Paper ( Held On 25 April 2013 )
A)
0.57 (M)
done
clear
B)
5.7 (M)
done
clear
C)
11.4 (M)
done
clear
D)
1.14 (M)
done
clear
View Answer play_arrow
-
question_answer28) The structure of which of the following chloro species can be explained on basis of \[ds{{p}^{2}}\] hybridization?
JEE Main Online Paper ( Held On 25 April 2013 )
A)
\[\operatorname{Pd}Cl_{4}^{2-}\]
done
clear
B)
\[\operatorname{Fe}Cl_{4}^{2-}\]
done
clear
C)
\[{{C}_{O}}Cl_{4}^{2-}\]
done
clear
D)
\[NiCl_{4}^{2-}\]
done
clear
View Answer play_arrow
-
question_answer29)
Which of the following reagent (s) used for the conversion 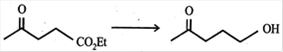 JEE Main Online Paper ( Held On 25 April 2013 )
JEE Main Online Paper ( Held On 25 April 2013 )
A)
\[\text{glycol}/\text{LiAI}{{\text{H}}_{4}}/{{\operatorname{H}}_{3}}{{\operatorname{O}}^{+}}\]
done
clear
B)
\[\text{glycol}/\text{NaH/}{{\operatorname{H}}_{3}}{{\operatorname{O}}^{+}}\]
done
clear
C)
\[\operatorname{L}\operatorname{i}\operatorname{A}{{\operatorname{IH}}_{4}}\]
done
clear
D)
\[{{\operatorname{NaBH}}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer30) A solution of copper sulphate\[({{\operatorname{CuSO}}_{4}})\]is electrolyzed for 10 minutes with a current of 1.5 ampers. The mass of copper deposited at the cathode (at. Mass of Cu=63u) is:
JEE Main Online Paper ( Held On 25 April 2013 )
A)
0.3892g
done
clear
B)
0.2938 g
done
clear
C)
0.2398 g
done
clear
D)
0.3928 g
done
clear
View Answer play_arrow
 In the above compounds correct order of reactivity in electrophilic substitution reactions will be:
JEE Main Online Paper ( Held On 25 April 2013 )
In the above compounds correct order of reactivity in electrophilic substitution reactions will be:
JEE Main Online Paper ( Held On 25 April 2013 )
 is
JEE Main Online Paper ( Held On 25 April 2013 )
is
JEE Main Online Paper ( Held On 25 April 2013 )
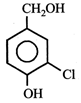
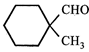
 JEE Main Online Paper ( Held On 25 April 2013 )
JEE Main Online Paper ( Held On 25 April 2013 )
