A) phenoxide ion is stabilized by resonance
B) phenols are more soluble in polar solvents
C) phenoxide ions do not exhibit resonance
D) alcohols do not lose H atoms at all
Correct Answer: A
Solution :
Phenol is more acidic than alcohol because phenoxide ion is stabilized by resonance.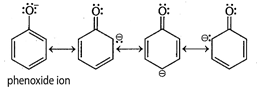 Resonance structure of phenoxide ion of phenol
Resonance structure of phenoxide ion of phenol
You need to login to perform this action.
You will be redirected in
3 sec
