A) +3
B) + 5
C) + 10
D) + 6
Correct Answer: D
Solution :
The structure of\[Cr{{O}_{5}}\]is as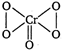 The structure shows that four oxygen atom in\[Cr{{O}_{5}}\]are involve in the formation of peroxide linkage (OS of oxygen = -1) while one is present as oxide (OS of oxygen = -2). Therefore, \[Cr{{O}_{5}}\] \[\underset{for\,\,Cr}{\mathop{x}}\,+1\times \underset{for\,\,O}{\mathop{(-2)}}\,+4\underset{for\,\,(O-O)}{\mathop{(-1)=0}}\,\] \[x-2-4=0\] \[x=\pm 6\]
The structure shows that four oxygen atom in\[Cr{{O}_{5}}\]are involve in the formation of peroxide linkage (OS of oxygen = -1) while one is present as oxide (OS of oxygen = -2). Therefore, \[Cr{{O}_{5}}\] \[\underset{for\,\,Cr}{\mathop{x}}\,+1\times \underset{for\,\,O}{\mathop{(-2)}}\,+4\underset{for\,\,(O-O)}{\mathop{(-1)=0}}\,\] \[x-2-4=0\] \[x=\pm 6\]
You need to login to perform this action.
You will be redirected in
3 sec
