A) \[{{n}_{1}}=1\,to\,{{n}_{2}}=2\]
B) \[{{n}_{1}}=2\,\,to\,\,{{n}_{2}}=1\]
C) \[{{n}_{1}}=2\,\,to\,\,{{n}_{2}}=6\]
D) \[{{n}_{1}}=6\,\,to\,\,{{n}_{2}}=2\]
Correct Answer: B
Solution :
As\[{{E}_{1}}>{{E}_{2}}\] \[\therefore \]\[{{v}_{1}}>{{v}_{2}}\]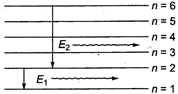 \[ie\], photons of higher frequency will be emitted if transition takes place from\[n=2\]to\[n=1\].
\[ie\], photons of higher frequency will be emitted if transition takes place from\[n=2\]to\[n=1\].
You need to login to perform this action.
You will be redirected in
3 sec
