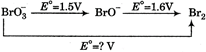 The unknown standard reduction potential is
The unknown standard reduction potential is
A) \[-1.6\,\,V\]
B) \[1.6\,\,V\]
C) \[-1.52\,\,V\]
D) \[1.52\ \,V\]
Correct Answer: D
Solution :
The given half reactions are \[BrO_{3}^{-}+4{{e}^{-}}+4{{H}^{+}}\xrightarrow{{}}{{E}^{o}}(in\,\,V)\Delta {{G}^{o}}=nF{{E}^{o}}\] \[Br{{O}^{-}}+2{{H}_{2}}O\] \[1.50\] \[-6F\] \[Br{{O}^{-}}+{{e}^{-}}+2{{H}^{+}}\xrightarrow{{}}\]\[1.60\] \[-1.6\,\,F\] \[\frac{1}{2}B{{r}_{2}}+{{H}_{2}}O\] Adding\[BrO_{3}^{-}+5{{e}^{-}}+6{{H}^{+}}\xrightarrow{{}}\frac{1}{2}B{{r}_{2}}+3{{H}_{2}}O\] \[\Delta {{G}^{o}}=-7.6F=-5{{E}^{o}}F\] \[{{E}^{o}}=\frac{7.6}{5}=1.52\,\,V\]You need to login to perform this action.
You will be redirected in
3 sec
