A) lone pair-bond pair repulsion only
B) bond pair-bond pair repulsion only
C) lone pair-lone pair repulsion and lone pair-bond pair repulsion
D) lone pair-lone pair repulsion only
Correct Answer: D
Solution :
In\[Br{{F}_{3}}\]molecule, Br is\[s{{p}^{3}}d\]hybrid, but geometry is T-shaped due to distortion of geometry from trigonal-bipyramidal to T-shaped by the involvement of lone pair-lone pair repulsion. Here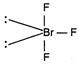 \[Ip-Ip\]repulsion\[=0\] \[Ip-bp\]repulsion\[=4\] \[bp-bp\]repulsion\[=2\]
\[Ip-Ip\]repulsion\[=0\] \[Ip-bp\]repulsion\[=4\] \[bp-bp\]repulsion\[=2\]
You need to login to perform this action.
You will be redirected in
3 sec
