A) \[Si{{F}_{4}}\]and \[S{{F}_{4}}\]
B) \[IO_{3}^{-}\]and\[Xe{{O}_{3}}\]
C) \[BH_{4}^{-}\]and \[NH_{4}^{+}\]
D) \[PF_{6}^{-}\]and\[S{{F}_{6}}\]
Correct Answer: A
Solution :
\[Si{{F}_{4}}\]and\[S{{F}_{4}}\]are not isostructural because\[Si{{F}_{4}}\]is tetrahedral due to\[s{{p}^{3}}-\]hybridisation of\[Si\]. \[_{14}Si=1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}}3{{p}^{2}}\] (In ground state)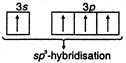 \[_{14}Si=1{{s}^{2}},2{{s}^{2}},3{{s}^{1}}3{{p}^{3}}\] (In excited state) Hence, four equivalent\[s{{p}^{3}}-\]hybrid orbitals are obtained and they are overlapped by four p-orbitals of four fluorine atoms on their axes. Thus it shows following structure
\[_{14}Si=1{{s}^{2}},2{{s}^{2}},3{{s}^{1}}3{{p}^{3}}\] (In excited state) Hence, four equivalent\[s{{p}^{3}}-\]hybrid orbitals are obtained and they are overlapped by four p-orbitals of four fluorine atoms on their axes. Thus it shows following structure 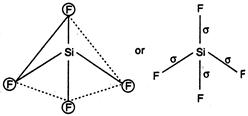 While\[S{{F}_{4}}\]is not tetrahedral but it is distorted tetrahedral because in it S is\[s{{p}^{3}}d\]hybrid. \[_{16}S=1{{s}^{2}},2{{p}^{6}},3{{s}^{2}}3p_{x}^{2}3p_{y}^{1}3p_{z}^{1}3p_{z}^{1}\] (In ground state) \[=1{{s}^{2}},2{{p}^{2}}2{{p}^{6}},3{{s}^{2}}3p_{x}^{1}3p_{y}^{1}3p_{z}^{1}3d_{xy}^{1}\] \[s{{p}^{3}}d-\]hybridisation (In first excitation state)
While\[S{{F}_{4}}\]is not tetrahedral but it is distorted tetrahedral because in it S is\[s{{p}^{3}}d\]hybrid. \[_{16}S=1{{s}^{2}},2{{p}^{6}},3{{s}^{2}}3p_{x}^{2}3p_{y}^{1}3p_{z}^{1}3p_{z}^{1}\] (In ground state) \[=1{{s}^{2}},2{{p}^{2}}2{{p}^{6}},3{{s}^{2}}3p_{x}^{1}3p_{y}^{1}3p_{z}^{1}3d_{xy}^{1}\] \[s{{p}^{3}}d-\]hybridisation (In first excitation state) 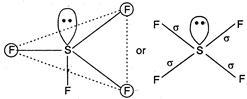 Hence, five\[s{{p}^{3}}d\]hybrid orbitals are obtained. One orbital is already paired and rest four are overlapped with four p-orbitals of four fluorine atoms on their axis in trigonal bipyramidal form. This structure is distorted from trigonal bi-pyramidal to tetrahedral due to involvement of repulsion between lone pair and bond pair.
Hence, five\[s{{p}^{3}}d\]hybrid orbitals are obtained. One orbital is already paired and rest four are overlapped with four p-orbitals of four fluorine atoms on their axis in trigonal bipyramidal form. This structure is distorted from trigonal bi-pyramidal to tetrahedral due to involvement of repulsion between lone pair and bond pair.
You need to login to perform this action.
You will be redirected in
3 sec
