A) isopropyi bromide
B) 3-bromo propane
C) allyl bromide
D) n-propyl bromide
Correct Answer: D
Solution :
Reaction of\[HBr\]with propene in the presence of peroxide gives n-propyl bromide. This addition reaction is an example of anti-Markownikoff addition reaction. (ie, it is completed in form of free radical .addition.) \[C{{H}_{3}}-CH=C{{H}_{2}}+HBr\xrightarrow[{}]{peroxide}\] \[\underset{n-propyl\text{ }bromide}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}Br}}\,\] Mechanism of this reaction is represented as follows Step 1. Formation of free radical of peroxide by means of decomposition. \[\underset{Benzoyl\text{ }peroxide}{\mathop{{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} |\,\,| \\ O \end{smallmatrix}}{\mathop{C}}\,-O-O-\underset{\begin{smallmatrix} |\,\,| \\ O \end{smallmatrix}}{\mathop{C}}\,-{{C}_{6}}{{H}_{5}}}}\,\xrightarrow[{}]{\Delta }\] \[\underset{Benzoate\text{ }free\text{ }radical}{\mathop{2{{C}_{6}}{{H}_{5}}-CO\overset{\bullet }{\mathop{O}}\,}}\,\] Step 2. Benzoate free radical forms bromine free radical with\[HBr\]. \[{{C}_{6}}{{H}_{5}}CO\overset{\bullet }{\mathop{O}}\,+HBr\xrightarrow[{}]{{}}{{C}_{6}}{{H}_{5}}COOH+\overset{\bullet }{\mathop{B}}\,r\] Step 3. Bromine free radical attacks on\[C=C\]of propene to form intermediate free radical.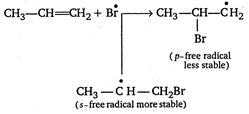 Hence,\[C{{H}_{3}}-\overset{\bullet }{\mathop{C}}\,H-C{{H}_{2}}Br\]is the major product of this step. Step 4. More stable free radical accept hydrogen free radical from benzoic acid and give final product of reaction. \[C{{H}_{3}}-\overset{\bullet }{\mathop{C}}\,H-C{{H}_{2}}B{{r}_{4}}-{{C}_{6}}{{H}_{5}}COOH\xrightarrow[{}]{{}}\] \[\underset{n-propyl\text{ }bromide}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}Br+{{C}_{6}}{{H}_{5}}CO\overset{\bullet }{\mathop{O}}\,}}\,\] Step 5. Benzoate free radicals are changed into benzoyl peroxide for the termination of free radical chain. \[{{C}_{6}}{{H}_{5}}CO\overset{\bullet }{\mathop{O}}\,+{{C}_{6}}{{H}_{5}}CO\overset{\bullet }{\mathop{O}}\,\xrightarrow[{}]{{}}{{({{C}_{6}}{{H}_{5}}CO)}_{2}}{{O}_{2}}\]
Hence,\[C{{H}_{3}}-\overset{\bullet }{\mathop{C}}\,H-C{{H}_{2}}Br\]is the major product of this step. Step 4. More stable free radical accept hydrogen free radical from benzoic acid and give final product of reaction. \[C{{H}_{3}}-\overset{\bullet }{\mathop{C}}\,H-C{{H}_{2}}B{{r}_{4}}-{{C}_{6}}{{H}_{5}}COOH\xrightarrow[{}]{{}}\] \[\underset{n-propyl\text{ }bromide}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}Br+{{C}_{6}}{{H}_{5}}CO\overset{\bullet }{\mathop{O}}\,}}\,\] Step 5. Benzoate free radicals are changed into benzoyl peroxide for the termination of free radical chain. \[{{C}_{6}}{{H}_{5}}CO\overset{\bullet }{\mathop{O}}\,+{{C}_{6}}{{H}_{5}}CO\overset{\bullet }{\mathop{O}}\,\xrightarrow[{}]{{}}{{({{C}_{6}}{{H}_{5}}CO)}_{2}}{{O}_{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
