A) 0.8 g/cc
B) 1.2 g/cc
C) 1.4 g/cc
D) 1.6 g/cc
Correct Answer: A
Solution :
As shown in figure, in the two arms of a tube pressure remains same on surface PP. Hence, \[8\times {{p}_{Y}}\times g+2\times {{p}_{Hg}}\times g=10\times {{p}_{x}}\times g\]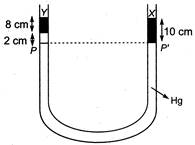 \[\therefore \] \[8{{p}_{Y}}+2\times 13.6=10\times 3.36\] Or \[{{p}_{Y}}=\frac{33.6-27.2}{8}\] \[=0.8g/cc\]
\[\therefore \] \[8{{p}_{Y}}+2\times 13.6=10\times 3.36\] Or \[{{p}_{Y}}=\frac{33.6-27.2}{8}\] \[=0.8g/cc\]
You need to login to perform this action.
You will be redirected in
3 sec
