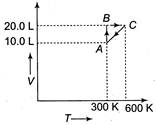
A) Isochoric
B) Isobaric
C) Isothermal
D) Cyclic
Correct Answer: B
Solution :
At\[A\to \]temperature =300 K, volume = 10 L, pressure\[={{p}_{1}}\] At\[C\to \]temperature = 600 K Volume = 20 L, Pressure\[={{p}_{2}}\] From \[\frac{{{p}_{1}}{{V}_{1}}}{{{T}_{1}}}=\frac{{{p}_{2}}{{V}_{2}}}{{{T}_{2}}}\] \[\frac{{{p}_{1}}\times 10}{300}=\frac{{{p}_{2}}\times 20}{600}\] or \[{{p}_{1}}={{p}_{2}}\] i.e., process is isobaric.You need to login to perform this action.
You will be redirected in
3 sec
