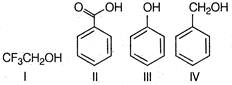
A) I<IV<III<II
B) III<IV<I<II
C) IV<I<III<II
D) II<III<I<IV
Correct Answer: C
Solution :
Benzoic acid is more acidic than phenol. because it is more resonance stabilised and the negative charge on the carboxylate ion is delocalised over two oxygen atoms.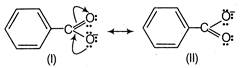 phenol is more acidic than \[C{{F}_{3}}C{{H}_{2}}OH\], because of its resonance stabilised phenoxide ion. Furthermore,\[C{{F}_{3}}C{{H}_{2}}OH\]is more acidic as compared to benzyl alcohol, because of the presence of more electron withdrawing groups. Thus, the correct order of acidity is
phenol is more acidic than \[C{{F}_{3}}C{{H}_{2}}OH\], because of its resonance stabilised phenoxide ion. Furthermore,\[C{{F}_{3}}C{{H}_{2}}OH\]is more acidic as compared to benzyl alcohol, because of the presence of more electron withdrawing groups. Thus, the correct order of acidity is 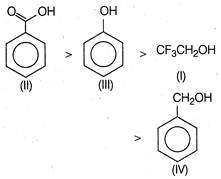
You need to login to perform this action.
You will be redirected in
3 sec
