A) -121 kJ per mol
B) +121 kJ per mol
C) +242 kJ per mol
D) -242 kJ per mol
Correct Answer: A
Solution :
[a] 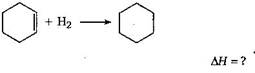 |
| \[\Delta H=\] [\[\Delta H\]of combustion of cyclohexane |
| - (\[\Delta H\] of combustion of cyclohexene |
| +\[\Delta H\]of combustion of H2)] |
| \[=-[-3920-(3800-24)]\,kJ\] |
| \[=-[3920+4041]\,kJ\] |
| = -[121] kJ |
| = -121 kJ |
You need to login to perform this action.
You will be redirected in
3 sec
