A) Acyl chloride > Acid anhydride > Ester > Amide
B) Ester > Acyl chloride > Amide >Acid anhydride
C) Acid anhydride > Amide, > Ester > Acyl chloride
D) Acyl chloride > Ester > Acid anhydride > Amide
Correct Answer: A
Solution :
| [a] Key Idea: The ease of nucleophilic substitution is depend upon the nature of leaving group. |
| When the leaving tendency of a group in a compound is high, then the compound is more reactive towards nucleophilic substitution. |
| The nucleophilic acyl substitution is completed in two steps as shown below |
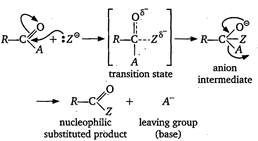 |
| The reactivity of the compound may be explained on the basicity of the leaving group. |
| A weaker base is a better leaving group. The basicity order is as: |
| \[C{{l}^{-}}>RCO{{O}^{-}}>R{{O}^{-}}>NH_{2}^{-}\] |
| Hence, the order of leaving tendency is \[C{{l}^{-}}>RCO{{O}^{-}}>R-{{O}^{-}}>NH_{2}^{-}\] and therefore, the order of reactivity of acyl compound is as: |
| |
| Acyl chloride > Acid anhydride > Ester > Amide |
You need to login to perform this action.
You will be redirected in
3 sec
