A) \[NO_{2}^{-}\]
B) \[S{{O}_{2}}\]
C) \[NO_{2}^{+}\]
D) \[{{O}_{3}}\]
Correct Answer: C
Solution :
| \[\overset{+}{\mathop{N}}\,{{O}_{2}}\] has linear shape due to sp-hybridisation of N of \[N{{O}_{2}}\] |
| \[O=\overset{+}{\mathop{N}}\,=O\] |
| While \[S{{O}_{2}},\,NO_{2}^{-}\] and \[{{O}_{3}}\] have angular shape |
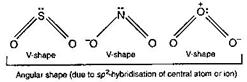 |
You need to login to perform this action.
You will be redirected in
3 sec
