A) \[[BC{{l}_{3}}\,\text{and}\,BrC{{l}_{3}}]\]
B) \[[N{{H}_{3}}\,\text{and}\,NO_{3}^{-}]\]
C) \[[N{{F}_{3}}\,\text{and}\,B{{F}_{3}}]\]
D) \[[BF_{4}^{-}\,\text{and}\,NH_{4}^{+}]\]
Correct Answer: D
Solution :
| If number of bond pairs and lone pairs are same for the given pairs, they are |
| is structural. |
| [a]\[BC{{l}_{3}}\]and\[BrC{{l}_{3}}\] |
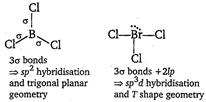 |
| [b]\[N{{H}_{3}}\,\text{and}\,NO_{3}^{-}\] |
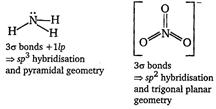 |
| [c]\[N{{F}_{3}}\,\text{and}\,BF_{3}^{{}}\] |
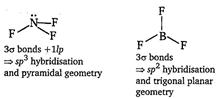 |
| [d]\[BF_{4}^{-}\,\text{and}\,NH_{4}^{+}\] |
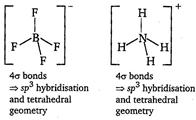 |
| Thus, \[BF_{4}^{-}\,\text{and}\,NH_{4}^{+}\]are isostructural. |
You need to login to perform this action.
You will be redirected in
3 sec
