A) The \[HCH\] bond angle in \[C{{H}_{4}},\] the \[HN\]bond angle in \[N{{H}_{3}},\] and the \[HOH\] bond angle in \[{{H}_{2}}O\] are all greater than \[{{90}^{o}}\]
B) The \[HOH\] bond angle in \[{{H}_{2}}O\] is larger than the \[HCH\] bond angle in \[C{{H}_{4}}\]
C) The \[HOH\] bond angle in \[{{H}_{2}}O\] is smaller than the x\[HNH\] bond angle in \[N{{H}_{3}}\]
D) The \[HCH\] bond angle in \[C{{H}_{4}}\] is larger than the \[HNH\] bond angle in \[N{{H}_{3}}\]
Correct Answer: B
Solution :
[b]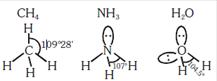
You need to login to perform this action.
You will be redirected in
3 sec
