| The reaction, [AIPMT (M) 2010] |
| \[2A(g)+B(g)3C(g)+D(g)\] |
| is begun with the concentrations of A and B both at an initial value of 1.00 M. When equilibrium is reached, the concentration of D is measured and found to be 0.25 M. The value for the equilibrium constant for this reaction is given by the expression |
A) \[[{{(0.75)}^{3}}(0.25)]\div [{{(1.00)}^{2}}(1.00)]\]
B) \[[{{(0.75)}^{3}}(0.25)]\div [{{(0.50)}^{2}}(0.75)]\]
C) \[[{{(0.75)}^{3}}(0.25)]\div [{{(0.50)}^{2}}(0.25)]\]
D) \[[{{(0.75)}^{3}}(0.25)]\div [{{(0.75)}^{2}}(0.25)]\]
Correct Answer: B
Solution :
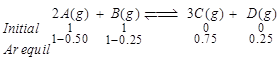
You need to login to perform this action.
You will be redirected in
3 sec
