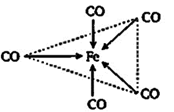
A) trigonal bipyramidal
B) octahedral
C) tetrahedral
D) square pyramidal
Correct Answer: A
Solution :
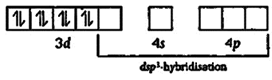
| [a] In, \[Fe{{(CO)}_{5}},\] the Fe atom is in \[ds{{p}^{3}}\] hybridised state. Therefore, the shape of molecule is trigonal bipyramidal. The hybridisation is as |
| \[_{26}Fe=1{{s}^{2}},\,\,2{{s}^{2}}\,2{{p}^{6}},\,3{{s}^{2}}\,3{{p}^{6}}\,3{{p}^{6}},\,4{{s}^{2}}\,4{{p}^{0}}\] |
| In \[Fe{{(CO)}_{5}}\] the \['Fe'\] atom is - |
 |
 |
You need to login to perform this action.
You will be redirected in
3 sec
