A) linkage isomerism
B) geometrical isomerism
C) coordination isomerism
D) ionisation isomerism
Correct Answer: B
Solution :
| [b] Key Idea Complexes of \[[M{{A}_{4}}{{B}_{2}}]\] type exhibit geometrical isomerism. |
| The complex \[{{[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]}^{+}}\]is a\[[M{{A}_{4}}{{B}_{2}}]\] type complex and thus, fulfills the conditions that are necessary to exhibit geometrical isomerism. |
| Hence, it has two geometrical isomers of different colours as: |
| The structure of the geometrical isomers is as |
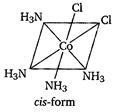 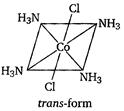 |
| For linkage isomerism, presence of ambidentate ligand is necessary. For coordination isomerism, both the cation and anion of the complex must be complex ions. For ionisation isomerism, an anion different to the ligands must be present outside the coordination sphere. All these conditions are not satisfied by this complex. Hence, it does not exhibit other given isomerisms. |
You need to login to perform this action.
You will be redirected in
3 sec
