A) isopropyl bromide
B) 3-bromo propane
C) allyl bromide
D) n-propyl bromide
Correct Answer: D
Solution :
| [d] Reaction of HBr with propene in the presence of peroxide gives n-propyl bromide. This addition reaction is an example of Anti-Markownikoff addition reaction. |
| (i.e., it is completed in form of free radical addition) |
| |
| |
| |
| Mechanism of this reaction is represented as follows: |
| Step 1. Formation of free radical of peroxide by means of decomposition. |
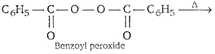 |
| Step 2. Benzoate free radical forms bromine |
| free radical with HBr. |
| |
| Step 3. Bromine free radical attacks on |
| of propene to form intermediate free radical. |
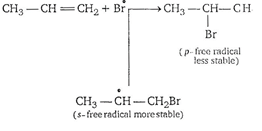 |
| Hence, |
| Step 4. More stable free radical accept hydrogen free radical from benzoic acid and give final product of reaction. |
| |
| |
| Step 5. Benzoate free radicals are changed into benzoyl peroxide for the termination of free radical chain. |
| |
You need to login to perform this action.
You will be redirected in
3 sec
