| "Metals are usually not found as nitrates in their ores". Out of the following two (a and b) reasons which is/are true for the above observation? |
| I. Metal nitrates are highly unstable. [NEET 2015 ] |
| II. Metal nitrates are highly soluble in water. |
A) I and II are true
B) I and II are false
C) I is false but II is true
D) I is true but II is false
Correct Answer: C
Solution :
| [c] Metals are usually not found as nitrates in their ores, because metal nitrates are highly, soluble in water. For example, \[KN{{O}_{3}}\] (salt peter) would be classified as completely soluble. Thus, \[KN{{O}_{3}}\] could be expected to dissociate completely in aqueous solution into \[{{K}^{+}}\]and \[NO_{3}^{-}\] ions |
| \[KN{{O}_{3}}{{K}^{+}}(aq)+NO_{3}^{-}(aq)\] |
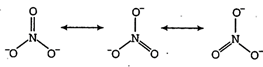
| The nitrate anion has three equivalent oxygen surrounding a central nitrogen atom. This tends to spread the single negative charge and make it easier for water (using hydrogen bonds) to separate the ions in solution. |
 |
You need to login to perform this action.
You will be redirected in
3 sec
