| (I) \[{{H}_{2}}{{O}_{2}}\,+{{O}_{3}}\,\xrightarrow{\,}\,{{H}_{2}}O\,+2{{O}_{2}}\] |
| (II) \[{{H}_{2}}{{O}_{2}}+A{{g}_{2}}O\to 2Ag+{{H}_{2}}O+{{O}_{2}}\] |
| Role of hydrogen peroxide in the above reactions is respectively [AIPMT 2014] |
A) oxidising in (I) and reducing in (II)
B) reducing in (I) and oxidizing in (II)
C) reducing in (I) and (II)
D) oxidising in (I) and (II)
Correct Answer: A
Solution :
| In the reaction, |
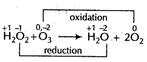 |
| Since \[{{H}_{2}}{{O}_{2}}\] oxidises, \[{{O}_{3}}\] into\[{{O}_{2}}\], thus it behaves as an oxidising agent. |
| Further, in the reaction, |
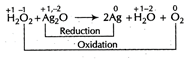 |
| Here \[{{H}_{2}}{{O}_{2}}\] reduces \[A{{g}_{2}}O\] into metallic silver [Ag] (as oxidation number is reducing from +1 to 0). Thus, \[{{H}_{2}}{{O}_{2}}\] behaves as a reducing agent. |
You need to login to perform this action.
You will be redirected in
3 sec
