A) \[{{S}_{2}}O_{4}^{2-}<{{S}_{2}}O_{6}^{2-}<SO_{3}^{2-}\]
B) \[{{S}_{2}}O_{6}^{2-}<{{S}_{2}}O_{4}^{2-}<SO_{3}^{2-}\]
C) \[{{S}_{2}}O_{4}^{2-}<SO_{3}^{2-}<{{S}_{2}}O_{6}^{2-}\]
D) \[SO_{3}^{2-}<{{S}_{2}}O_{4}^{2-}<{{S}_{2}}O_{6}^{2-}\]
Correct Answer: C
Solution :
| Oxidation state of \['S'\] in |
| \[SO_{3}^{2-},\,\,x+\,(-2\times 3)=-2,\,\,x=+6-2=+4\] |
| Oxidation state of \['S'\] in |
| \[{{S}_{2}}O_{4}^{2-}\,2x\,+(-2\times 4)=-2\] |
| \[2x=+8-2=+\,6\] |
| \[x=\frac{+6}{2}=+3\] |
| Oxidation state of \['S'\] in |
| \[{{S}_{2}}O_{6}^{2-}\,2x+(-2\times 6)=-2\] |
| \[2x=12-2=10\] |
| \[x=\frac{10}{2}=+5\] |
| On the basis of structures |
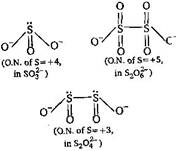 |
| Hence, increasing order of oxidation state of is |
| \[{{S}_{2}}O_{4}^{2-}<SO_{3}^{2-}<{{S}_{2}}O_{6}^{2-}\] |
You need to login to perform this action.
You will be redirected in
3 sec
