| The aqueous solution containing which one of the following ions will be colourless? [AIPMT (S) 2005] |
| (Atomic no. : Sc = 21, Fe = 26, Ti = 22, Mn = 25) |
A) \[S{{c}^{3+}}\]
B) \[F{{e}^{2+}}\]
C) \[T{{i}^{3+}}\]
D) \[M{{n}^{2+}}\]
Correct Answer: A
Solution :
| [a] \[_{21}Sc=1{{s}^{2}},2{{s}^{2}}\,2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}\,3{{d}^{1}},\,4{{s}^{2}}\] |
| So, \[S{{c}^{3+}}=1{{s}^{2}},2{{s}^{2}}s{{p}^{6}},3{{s}^{2}}3{{p}^{6}}\] |
| (It is colourless due to absence of unpaired electrons in d-subshell) |
| \[_{26}Fe=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{6}},4{{s}^{2}}\] |
| \[F{{e}^{2+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},\,3{{s}^{2}}3{{p}^{6}}3{{d}^{6}}\] |
| |
| (It is coloured due to presence of four unpaired electrons in d-subshell) |
| \[\begin{align} & _{22}Ti=1{{s}^{2}},2{{s}^{2}}\,2{{p}^{6}},\,3{{s}^{2}}3{{p}^{6}}3{{d}^{2}},4{{s}^{2}} \\ & T{{i}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{1}} \\ \end{align}\] |
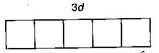 |
| (It is coloured due to presence of an unpaired electron in d-subshell) |
| \[\begin{align} & _{25}Mn=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{5}},4{{s}^{2}} \\ & M{{n}^{2+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{5}} \\ \end{align}\] |
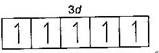 |
| (It is coloured due to presence of unpaired electrons in d-subshell). |
You need to login to perform this action.
You will be redirected in
3 sec
