A) The \[\pi \]-bonding involves overlap of p-orbitals of oxygen with p-orbtials of manganese
B) The \[\pi \]-bonding involves overlap of d-orbitals of oxygen with d-orbitals of manganese
C) The \[\pi \]-bonding involves overlap of p-orbitals of oxygen with d-orbitals of manganese
D) There is no \[\pi \]-bonding
Correct Answer: C
Solution :
| [c] Structure of permanganate ion |
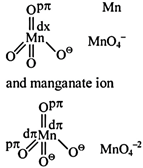 |
You need to login to perform this action.
You will be redirected in
3 sec
