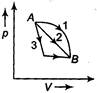
| An ideal gas goes from state A to state B via three different processes as indicated in the p-V diagram. If \[{{Q}_{1}},\,\,{{Q}_{2}},\,\,{{Q}_{3}}\] indicate the heat absorbed by the gas along the three processes and \[\Delta {{U}_{1}},\Delta {{U}_{2}},\Delta {{U}_{3}}\] indicate the change in internal energy along the three processes respectively, then [AIPMT (M) 2012] |
 |
A) \[{{Q}_{1}}>{{Q}_{2}}>{{Q}_{3}}\] and \[\Delta {{U}_{1}}=\Delta {{U}_{2}}=\Delta {{U}_{3}}\]
B) \[{{Q}_{3}}>{{Q}_{2}}>{{Q}_{1}}\] and \[\Delta {{U}_{1}}=\Delta {{U}_{2}}=\Delta {{U}_{3}}\]
C) \[{{Q}_{1}}={{Q}_{2}}={{Q}_{3}}\] and \[\Delta {{U}_{1}}>\Delta {{U}_{2}}>\Delta {{U}_{3}}\]
D) \[{{Q}_{3}}>{{Q}_{2}}>{{Q}_{1}}\] and \[\Delta {{U}_{1}}>\Delta {{U}_{2}}>\Delta {{U}_{3}}\]
Correct Answer: A
Solution :
| For all process 1, 2 and 3 |
| \[\Delta U={{U}_{B}}-{{U}_{A}}\] is same |
| \[\therefore \] \[\Delta {{U}_{1}}=\Delta {{U}_{2}}=\Delta {{U}_{3}}\] |
| Now, \[\Delta Q=\Delta U+\Delta W\] |
| Now, \[\Delta W=\]work done by the gas |
| \[\therefore \] \[\Delta {{Q}_{1}}=\Delta {{Q}_{2}}>\Delta {{Q}_{3}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
