| Figure below shows two paths that may be taken by a gas to go from a state A to a state C. |
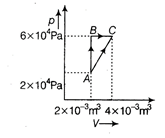 |
| In process AB, 400 J of heat is added to the system and in process BC, 100 J of heat is added to the system. The heat absorbed by the system in the process AC will be [NEET 2015] |
A) 380 J
B) 500 J
C) 460 J
D) 300 J
Correct Answer: C
Solution :
| Since, initial and final points are same |
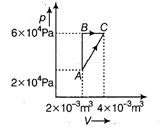 |
| So, \[\Delta {{U}_{A\to B\to C}}=\Delta {{U}_{A\to C}}\] (i) |
| Also \[A\to B\] is isochoric process |
| So \[d{{W}_{A\to B}}=0\] |
| and \[dQ=dU+dW\] |
| So, \[d{{Q}_{A\to B}}=d{{U}_{A\to B}}=400J\] |
| Next \[B\to C\] is isobaric process |
| So, \[d{{O}_{B\to C}}=d{{U}_{B\to C}}+d{{W}_{B\to C}}\] |
| \[=d{{U}_{B\to C}}+p\Delta {{V}_{B\to C}}\] |
| \[\Rightarrow \] \[100=d{{U}_{B\to C}}+6\times {{10}^{4}}(2\times {{10}^{-3}})\] |
| \[\Rightarrow \] \[D{{U}_{B\to C}}=100-120=-20J\] |
| From Eq. (i), |
| \[\because \] \[\Delta {{U}_{A\to B\to C}}=\Delta {{U}_{A\to C}}\] |
| \[\Rightarrow \] \[\Delta {{U}_{A\to B}}+\Delta {{U}_{B\to C}}=d{{Q}_{A\to C}}-d{{W}_{A\to C}}\] |
| \[\Rightarrow \] \[400+\left( -20 \right)=d{{Q}_{A\to C}}\] |
| \[-(p\Delta {{V}_{A}}+Area\,of\,\Delta ABC)\] |
| \[\Rightarrow \] \[d{{Q}_{A\to C}}=380+\left( \begin{align} & 2\times {{10}^{4}}\times 2\times {{10}^{-3}} \\ & +\frac{1}{2}\times 2\times {{10}^{-3}}\times 4\times {{10}^{4}} \\ \end{align} \right)\] |
| \[=380+(40+40)\] |
| \[d{{Q}_{A\to C}}=460J\] |
You need to login to perform this action.
You will be redirected in
3 sec
