A) \[(T+2.4)\text{ }K\]
B) \[(T-2.4)\text{ }K\]
C) \[(T+4)\text{ }K\]
D) \[(T-4)\text{ }K\]
Correct Answer: D
Solution :
| Key Idea: in an adiabatic process, there is no heat transfer into or out of a system i.e., \[Q=0\] |
| In an adiabatic process \[Q=0\] |
| So, from 1st law of thermodynamics. |
| \[W=-\Delta U\] |
| \[=-n{{C}_{V}}\Delta T\] |
| \[=-\left( \frac{R}{\gamma -1} \right)({{T}_{f}}-{{T}_{i}})\] |
| \[=\frac{nR}{\gamma -1}({{T}_{i}}-{{T}_{f}})\] |
| Here: \[W=6R\,\,\,J,\,\,n=1\,\,\,mol,\] |
| \[R=8.31\,J/mol-K,\,\,\gamma =\frac{5}{3},{{T}_{i}}=T\,K\] |
| Substituting given values in Eq. (i), we get |
| \[\therefore \] \[6R=\frac{R}{(5/3-1)}(T-{{T}_{f}})\] |
| \[\Rightarrow \] \[6R=\frac{3R}{2}(T-{{T}_{f}})\] |
| \[\Rightarrow \] \[T-{{T}_{f}}=4\] |
| \[\therefore \] \[{{T}_{f}}=(T-4)K\] |
| Note: Adiabatic expansions of mono, dia and polyatomic gases are shown below |
| 1\[\to \] monoatomic |
| 2\[\to \] diatomic |
| 3\[\to \] polyatomic |
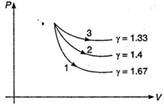 |
You need to login to perform this action.
You will be redirected in
3 sec
