| The dissociation equilibrium of a gas \[A{{B}_{2}}\] can be represented as [AIPMT (S) 2008] |
| \[2A{{B}_{2}}(g)2AB(g)+{{B}_{2}}(g)\] |
| The degree of dissociation is \['x'\] and is small compared to 1. The expression relating the degree of dissociation \[(x)\] with equilibrium constant \[{{K}_{p}}\] and total pressure p is |
A) \[(2{{K}_{p}}/p)\]
B) \[{{(2{{K}_{p}}/p)}^{1/3}}\]
C) \[{{(2{{K}_{p}}/p)}^{1/2}}\]
D) \[({{K}_{p}}/p)\]
Correct Answer: B
Solution :
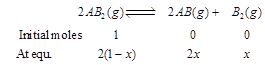 where, x = degree of dissociation where, x = degree of dissociation |
| Total moles at equilibrium |
| |
| So, |
| |
| |
| |
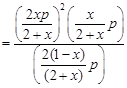 |
| |
| |
| |
You need to login to perform this action.
You will be redirected in
3 sec
