| The values of \[{{K}_{{{p}_{1}}}}\] and \[{{K}_{{{p}_{2}}}}\] for the reactions [AIPMT (S) 2008] |
| \[XY+Z\] ...(i) |
| and \[A2B\] ..(ii) |
| are in ratio of 9 : 1. If degree of dissociation of X and A be equal, then total pressure at equilibrium (i) and (ii) are in the ratio |
A) 3 : 1
B) 1 : 9
C) 36 : 1
D) 1 : 1
Correct Answer: C
Solution :
| From equation, |
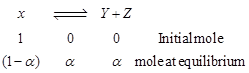 |
| |
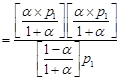 |
| |
| From equation |
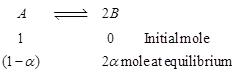 |
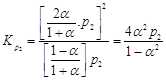 (ii)
(ii) |
| From Eqs (i) and (ii) |
| |
You need to login to perform this action.
You will be redirected in
3 sec
