A) \[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-O-C{{H}_{3}}\]
B) \[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-O-C{{H}_{3}}\]
C) \[\text{C}{{\text{H}}_{\text{3}}}\text{-}\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{C}{{\text{H}}_{\text{3}}} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,\text{-O-C}{{\text{H}}_{\text{3}}}\]
D) \[\text{C}{{\text{H}}_{\text{3}}}\text{-}\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{C}{{\text{H}}_{\text{3}}} \end{smallmatrix}}{\mathop{\text{CH}}}\,\text{-C}{{\text{H}}_{\text{2}}}\text{-O-C}{{\text{H}}_{\text{3}}}\]
Correct Answer: C
Solution :
The ether, which gives more stable carbonation, gives \[C{{H}_{3}}OH\] as one of the product with hot concentrated HI. The order of stability of carbonation is \[3{}^\circ >2{}^\circ >1{}^\circ \] Thus,\[\text{C}{{\text{H}}_{\text{3}}}-\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{C}{{\text{H}}_{\text{3}}} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,-\text{OC}{{\text{H}}_{\text{3}}}\] gives \[\text{C}{{\text{H}}_{\text{3}}}\text{OH}\] as one of the reaction. The reaction proceeds as \[{{\text{H}}_{\text{3}}}\text{C}-\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{C}{{\text{H}}_{\text{3}}} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,-\text{O}-\text{C}{{\text{H}}_{\text{3}}}\text{+}{{\text{H}}^{\text{+}}}\]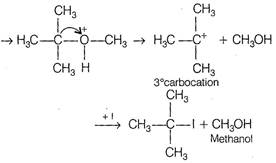
You need to login to perform this action.
You will be redirected in
3 sec
