A) \[C{{O}_{2}}\]
B) \[C{{H}_{4}}\]
C) \[N{{H}_{3}}\]
D) \[N{{F}_{3}}\]
Correct Answer: C
Solution :
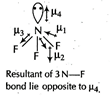
\[C{{O}_{2}}\]and \[C{{H}_{4}}\] have zero dipole moment as these are symmetrical in nature. Between \[N{{H}_{3}}\] and \[N{{F}_{3}},\,N{{F}_{3}}\] has greater dipole moment though in \[N{{H}_{3}}\]and \[N{{H}_{3}}\]both, N possesses one lone pair of electrons. This is because in case of \[N{{H}_{3}}\], the net N?H bond dipole is in the same direction as the direction of dipole of lone pair but in case of \[N{{H}_{3}}\], the direction of net bond dipole of three ?N?F bonds is opposite the that of the dipole of the lone pair.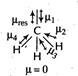 \[\because \]\[{{\mu }_{res}}={{\mu }_{1}}+{{\mu }_{2}}+{{\mu }_{3}}=-{{\mu }_{res}}=-{{\mu }_{4}}=0\] \[\therefore \]\[{{\mu }_{net}}={{\mu }_{1}}+{{\mu }_{2}}+{{\mu }_{3}}+{{\mu }_{4}}\] \[=-{{\mu }_{4}}+{{\mu }_{4}}=0\]
\[\because \]\[{{\mu }_{res}}={{\mu }_{1}}+{{\mu }_{2}}+{{\mu }_{3}}=-{{\mu }_{res}}=-{{\mu }_{4}}=0\] \[\therefore \]\[{{\mu }_{net}}={{\mu }_{1}}+{{\mu }_{2}}+{{\mu }_{3}}+{{\mu }_{4}}\] \[=-{{\mu }_{4}}+{{\mu }_{4}}=0\] 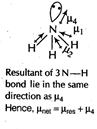
You need to login to perform this action.
You will be redirected in
3 sec
