A) \[C{{H}_{3}}N_{2}^{+}{{X}^{-}}\]
B) \[{{C}_{2}}{{H}_{5}}N_{2}^{+}{{X}^{-}}\]
C) \[C{{H}_{3}}C{{H}_{2}}N_{2}^{+}{{X}^{-}}\]
D) \[{{C}_{6}}{{H}_{5}}C{{H}_{2}}N_{2}^{+}{{X}^{-}}\]
Correct Answer: B
Solution :
Diazonium salt containing aryl group directly linked to the nitrogen atom is most stable due to resonance stabilization between the benzene nucleus and N-atom.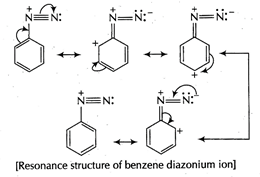
You need to login to perform this action.
You will be redirected in
3 sec
