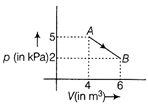
 The change in internal energy of the gas during the transition is
The change in internal energy of the gas during the transition is
A) 20 kJ
B) -20 kJ
C) 20 J
D) -12 kJ
Correct Answer: B
Solution :
For a diatomic gas,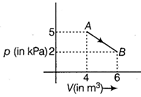 \[{{C}_{V}}=\frac{5}{2}R\] The change in internal energy of gas in the transition from A to B is \[\Delta U=n{{C}_{v}}dT\] \[=n\left( \frac{5R}{2} \right)({{T}_{B}}-{{T}_{A}})\] \[=nR\frac{5}{2}\left( \frac{{{p}_{B}}{{V}_{B}}}{nR}\frac{{{p}_{A}}{{V}_{A}}}{nR} \right)\] \[=\frac{5}{2}(2\times {{10}^{3}}\times 6-5\times {{10}^{3}}\times 4)\] \[=\frac{5}{2}\times (-8\times {{10}^{3}})\] \[=-\frac{4\times {{10}^{4}}}{2}=-20KJ\]
\[{{C}_{V}}=\frac{5}{2}R\] The change in internal energy of gas in the transition from A to B is \[\Delta U=n{{C}_{v}}dT\] \[=n\left( \frac{5R}{2} \right)({{T}_{B}}-{{T}_{A}})\] \[=nR\frac{5}{2}\left( \frac{{{p}_{B}}{{V}_{B}}}{nR}\frac{{{p}_{A}}{{V}_{A}}}{nR} \right)\] \[=\frac{5}{2}(2\times {{10}^{3}}\times 6-5\times {{10}^{3}}\times 4)\] \[=\frac{5}{2}\times (-8\times {{10}^{3}})\] \[=-\frac{4\times {{10}^{4}}}{2}=-20KJ\]
You need to login to perform this action.
You will be redirected in
3 sec
