A) \[HCO_{3}^{-}\]
B) \[Xe{{O}_{4}}\]
C) \[{{(CN)}_{2}}\]
D) \[C{{H}_{2}}{{(CN)}_{2}}\]
Correct Answer: B
Solution :
| Structure | \[\sigma \] and \[\pi \] bonds | |
| (a) | 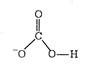 | \[\sigma \] bond - 4 \[\pi \] bond-1 |
| (b) | 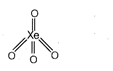 | \[\sigma \]bond-4 \[\pi \] bond-4 |
| (c) | \[N\equiv C-C\equiv N\] | \[\sigma \] bond-3 \[\pi \] bond-4 |
| (d) | \[N\equiv 2C-\underset{\begin{smallmatrix} \,\,| \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ \,\,| \end{smallmatrix}}{\mathop{C}}}\,-C\equiv N\] | \[\sigma \] bond-6 \[\pi \] bond-4 |
You need to login to perform this action.
You will be redirected in
3 sec
