A) In \[K\,vsT\]
B) \[\frac{\text{ln}K}{T}vsT\]
C) In \[Kvs\frac{l}{t}\]
D) \[\frac{T}{\ln \,k}vs\frac{l}{T}\]
Correct Answer: C
Solution :
By Arrhenius equation \[K=A{{e}^{-{{E}_{a}}/RT}}\] where, \[{{E}_{a}}\] = energy of activation Applying log on both the side, \[\ln \,k=\ln A-\frac{{{E}_{a}}}{RT}\,\,\,\,\,\,\,\] ?(i) or\[\log \,k=-\frac{{{E}_{a}}}{2.303RT}+\log A\,\] ?(ii) This equation is of the form of \[y=mx+c\] i.e. the equation of a straight line. Thus, if a plote of \[\log k\,vs\frac{1}{T}\] is a straight line, the validity of the equation is confirmed. Slope of the line \[=-\frac{{{E}_{a}}}{2.303R}\] Thus, measuring the slope of the line, the value of \[{{E}_{a}}\] can be calculated.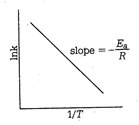
You need to login to perform this action.
You will be redirected in
3 sec
