A) \[SiC{{l}_{4}},PCl_{4}^{+}\]
B) Diamond, carbide
C) \[N{{H}_{3}},P{{H}_{3}}\]
D) \[Xe{{F}_{4}},Xe{{O}_{4}}\]
Correct Answer: D
Solution :
(i) Structure of \[\text{SiC}{{\text{l}}_{\text{4}}}\]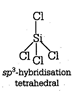 Structure of \[\text{PCl}_{\text{4}}^{\text{+}}\]
Structure of \[\text{PCl}_{\text{4}}^{\text{+}}\] 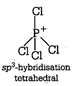 (ii) Diamond and silicon carbide \[(SiC),\] both are isostructural because their central atom is \[s{{p}^{3}}\] hybridized and both have tetrahedral arrangement. (iii) Structure of \[N{{H}_{3}}\]
(ii) Diamond and silicon carbide \[(SiC),\] both are isostructural because their central atom is \[s{{p}^{3}}\] hybridized and both have tetrahedral arrangement. (iii) Structure of \[N{{H}_{3}}\] 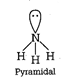 Structure of \[P{{H}_{3}}\]
Structure of \[P{{H}_{3}}\] 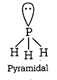 Both \[N{{H}_{3}}\] and \[P{{H}_{3}}\] have \[s{{p}^{3}}\] geometry. (iv) \[Xe{{F}_{4}}\] has \[s{{p}^{3}}{{d}^{2}}\] hybridisation while \[Xe{{O}_{4}}\] has \[s{{p}^{3}}\] hybridisation. Structure of \[Xe{{F}_{4}}\]
Both \[N{{H}_{3}}\] and \[P{{H}_{3}}\] have \[s{{p}^{3}}\] geometry. (iv) \[Xe{{F}_{4}}\] has \[s{{p}^{3}}{{d}^{2}}\] hybridisation while \[Xe{{O}_{4}}\] has \[s{{p}^{3}}\] hybridisation. Structure of \[Xe{{F}_{4}}\] 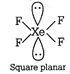 Structure of \[Xe{{O}_{4}}\]
Structure of \[Xe{{O}_{4}}\] 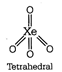 Hence, \[Xe{{F}_{4}}\] and \[Xe{{O}_{4}}\] are not isostructural.
Hence, \[Xe{{F}_{4}}\] and \[Xe{{O}_{4}}\] are not isostructural.
You need to login to perform this action.
You will be redirected in
3 sec
