A) Adiabatic
B) Isobaric
C) Isochoric
D) Isothermal
Correct Answer: C
Solution :
Given, ideal gas is compressed to half its initial volume i.e. \[{{V}_{0}}=\frac{V}{2}\]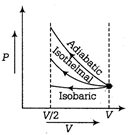 The isochoric process is one in which volume is kept constant, meaning that work done by the system will be zero i.e. \[{{W}_{isochoric}}=0\] As we know, work dine on the gas = Area under curve i.e. \[{{W}_{ediabatic}}>{{W}_{isothermal}}>{{W}_{isochoric}}\]
The isochoric process is one in which volume is kept constant, meaning that work done by the system will be zero i.e. \[{{W}_{isochoric}}=0\] As we know, work dine on the gas = Area under curve i.e. \[{{W}_{ediabatic}}>{{W}_{isothermal}}>{{W}_{isochoric}}\]
You need to login to perform this action.
You will be redirected in
3 sec
