A) presence of one \[-OH\] group and two \[P-H\] bonds
B) high electron gain enthalpy of phosphorus
C) high oxidation state of phosphorus
D) presence of two \[-OH\] groups and one \[P-H\] bond
Correct Answer: A
Solution :
The oxy acid of phosphorus which contain \[P-H\] bond act as a reducing agent or reductant.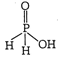 In \[{{H}_{3}}P{{O}_{2}}\] one \[-OH\] group and two \[P-H\] bonds are present.
In \[{{H}_{3}}P{{O}_{2}}\] one \[-OH\] group and two \[P-H\] bonds are present.
You need to login to perform this action.
You will be redirected in
3 sec
