A) \[1.26\times {{10}^{-5}}M\]
B) \[1.6\times {{10}^{-11}}M\]
C) \[1.6\times {{10}^{-9}}M\]
D) Zero
Correct Answer: B
Solution :
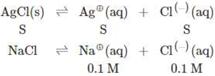 \[\therefore {{K}_{sp}}(AgCl)=S(S+0.1)\] \[\because S\ll 0.1\] \[\therefore S+0.1<<0.1\] \[\therefore 1.6\times {{10}^{-10}}=S=0.1\] \[\therefore S=1.6\times {{10}^{-9}}M\]
\[\therefore {{K}_{sp}}(AgCl)=S(S+0.1)\] \[\because S\ll 0.1\] \[\therefore S+0.1<<0.1\] \[\therefore 1.6\times {{10}^{-10}}=S=0.1\] \[\therefore S=1.6\times {{10}^{-9}}M\]
You need to login to perform this action.
You will be redirected in
3 sec
