A) Four
B) Two
C) One
D) Three
Correct Answer: B
Solution :
The structure of \[\text{CI}{{\text{F}}_{\text{3}}}\] is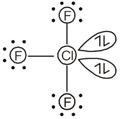 The number of lone pair of electrons on central Cl is 2.
The number of lone pair of electrons on central Cl is 2.
You need to login to perform this action.
You will be redirected in
3 sec
