A)
\[\text{MnO}_{\text{4}}^{\text{-}}\] \[{{\text{C}}_{\text{2}}}\text{O}_{\text{4}}^{\text{2-}}\] \[{{\text{H}}^{+}}\] 2 16 5
B)
\[\text{MnO}_{\text{4}}^{\text{-}}\] \[{{\text{C}}_{\text{2}}}\text{O}_{\text{4}}^{\text{2-}}\] \[{{\text{H}}^{+}}\] 2 5 16
C)
\[\text{MnO}_{\text{4}}^{\text{-}}\] \[{{\text{C}}_{\text{2}}}\text{O}_{\text{4}}^{\text{2-}}\] \[{{\text{H}}^{+}}\] 16 5 2
D)
\[\text{MnO}_{\text{4}}^{\text{-}}\] \[{{\text{C}}_{\text{2}}}\text{O}_{\text{4}}^{\text{2-}}\] \[{{\text{H}}^{+}}\] 5 16 2
Correct Answer: B
Solution :
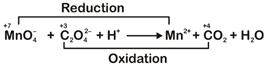 n-factor of \[\text{MnO}_{4}^{-}\Rightarrow 5\] n-factor of \[{{C}_{2}}O_{4}^{2-}\Rightarrow 2\] Ratio of n-factors of \[\text{MnO}_{\text{4}}^{\text{-}}\] and \[{{\text{C}}_{\text{2}}}\text{O}_{\text{4}}^{\text{2-}}\] is \[\text{5:2}\]So, molar ratio in balanced reaction is \[2:5\] \[\therefore \] The balanced equation is \[\text{2MnO}_{\text{4}}^{\text{-}}\text{+5}{{\text{C}}_{\text{2}}}\text{O}_{\text{4}}^{\text{2-}}\text{+16}{{\text{H}}^{\text{+}}}\to \text{2M}{{\text{n}}^{\text{2+}}}\text{+10C}{{\text{O}}_{\text{2}}}\text{+8}{{\text{H}}_{\text{2}}}\text{O}\]
n-factor of \[\text{MnO}_{4}^{-}\Rightarrow 5\] n-factor of \[{{C}_{2}}O_{4}^{2-}\Rightarrow 2\] Ratio of n-factors of \[\text{MnO}_{\text{4}}^{\text{-}}\] and \[{{\text{C}}_{\text{2}}}\text{O}_{\text{4}}^{\text{2-}}\] is \[\text{5:2}\]So, molar ratio in balanced reaction is \[2:5\] \[\therefore \] The balanced equation is \[\text{2MnO}_{\text{4}}^{\text{-}}\text{+5}{{\text{C}}_{\text{2}}}\text{O}_{\text{4}}^{\text{2-}}\text{+16}{{\text{H}}^{\text{+}}}\to \text{2M}{{\text{n}}^{\text{2+}}}\text{+10C}{{\text{O}}_{\text{2}}}\text{+8}{{\text{H}}_{\text{2}}}\text{O}\]
You need to login to perform this action.
You will be redirected in
3 sec
