A)
The electronic configuration of N atom is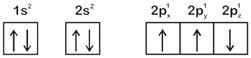
B) An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers
C) Total orbital angular momentum of electron in 's' orbital is equal to zero
D) The value of m for \[{{\text{d}}_{\text{z}}}\text{2}\] is zero
Correct Answer: A
Solution :
According to Hund's Rule of maximum multiplicity, the correct electronic configuration of N-atom is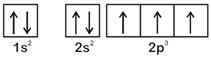 OR
OR 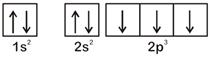 \[\therefore \] Option [a] violates Hund's Rule.
\[\therefore \] Option [a] violates Hund's Rule.
You need to login to perform this action.
You will be redirected in
3 sec
