-
question_answer1) The speed of a boat is 5 km/h in still water. It crosses a river of width 1.0 km along the shortest possible path in 15 min. The velocity of the river water is: (in km/h)
A)
5
done
clear
B)
1
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
-
question_answer2)
Two particles A and B are connected by a rigid rod AB. The rod slides along perpendicular rails as shown here. The velocity of A to the right is 10 m/s. What is the velocity of B when angle \[\alpha ={{60}^{o}}\]? 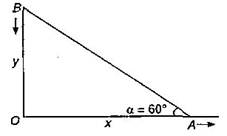
A)
9.8 m/s
done
clear
B)
10 m/s
done
clear
C)
5.8 m/s
done
clear
D)
17.3 m/s
done
clear
View Answer play_arrow
-
question_answer3) A mass of 1 kg is suspended by a thread. It is (i) lifted up with an acceleration \[4.9\,m/{{s}^{2}},\] (ii) lowered with an acceleration \[4.9\,m/{{s}^{2}}\]. The ratio of the tensions is:
A)
3 : 1
done
clear
B)
1 : 3
done
clear
C)
1 : 2
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
-
question_answer4) A car moving with a speed of 40 km/h can be stopped by applying brakes after at least 2 m. If me same car is moving with a speed of 80 km/h, what is the minimum stopping distance?
A)
8 m
done
clear
B)
2 m
done
clear
C)
4 m
done
clear
D)
6 m
done
clear
View Answer play_arrow
-
question_answer5) A force acts on a 3.0 g particle in such a way that the position of the particle as a function of time is given by \[x=3t-4{{t}^{2}}+{{t}^{3}}\], where \[x\] is in metre and \[t\] in second. The work done during the first 4 s is:
A)
570 mJ
done
clear
B)
450 mJ
done
clear
C)
490 mJ
done
clear
D)
528 mJ
done
clear
View Answer play_arrow
-
question_answer6) A bullet is fired from a gun. The force on the bullet is given by: \[F=600\,-2\times {{10}^{5}}t\] where F is in newton and t in second. The force on the bullet becomes zero as soon as it leaves the barrel. What is the average impulse imparted to the bullet?
A)
8 Ns
done
clear
B)
Zero
done
clear
C)
0.9 Ns
done
clear
D)
1.8 Ns
done
clear
View Answer play_arrow
-
question_answer7) Two equal masses \[{{m}_{1}}\] and \[{{m}_{2}}\] moving along the same straight line with velocities + 3 m/s and ?5 m/s respectively collide elastically. Their velocities after the collision will be respectively:
A)
+ 4 m/s for both
done
clear
B)
- 3m/s and + 5 m/s
done
clear
C)
- 4 m/s and + 4 m/s
done
clear
D)
- 5 m/s and + 3 m/s
done
clear
View Answer play_arrow
-
question_answer8) A 5000 kg rocket is set for vertical firing. The exhaust speed is \[800\,m{{s}^{-1}}\]. To give an initial upward acceleration of \[20\,m/{{s}^{2}},\] the amount of gas ejected per second to supply the needed thrust will be: \[(g=10\,m{{s}^{-2}})\]
A)
\[127.5\,\,kg\,{{s}^{-1}}\]
done
clear
B)
\[187.\,5\,kg\,{{s}^{-1}}\]
done
clear
C)
\[185.5\,kg\,{{s}^{-1}}\]
done
clear
D)
\[137.5\,kg\,{{s}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer9) A ball of mass 0.25 kg attached to the end of a string of length 1.96 m is moving in a horizontal circle. The string will break if the tension is more than 25 N. What is the maximum speed with which the ball can be moved?
A)
14 m/s
done
clear
B)
3 m/s
done
clear
C)
3.92 m/s
done
clear
D)
5 m/s
done
clear
View Answer play_arrow
-
question_answer10)
O is the centre of an equilateral triangle ABC. \[{{F}_{1}},\,{{F}_{2}}\] and \[{{F}_{3}}\] are three forces acting along the sides AB, BC and AC as shown in figure. What should be the magnitude of \[{{F}_{3}}\] so that the total torque about O is zero? 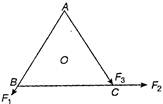
A)
\[({{F}_{1}}+{{F}_{2}})/2\]
done
clear
B)
\[({{F}_{1}}-{{F}_{2}})\]
done
clear
C)
\[({{F}_{1}}+{{F}_{2}})\]
done
clear
D)
\[2\,({{F}_{1}}+{{F}_{2}})\]
done
clear
View Answer play_arrow
-
question_answer11)
A weightless ladder 20 ft long rests against a frictionless wall at an angle of 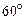 from the horizontal. A 150 pound man is 4 ft from the top of the ladder. A horizontal force is needed to keep it from slipping. Choose the correct magnitude of force from the following:
from the horizontal. A 150 pound man is 4 ft from the top of the ladder. A horizontal force is needed to keep it from slipping. Choose the correct magnitude of force from the following:
A)
17.3 pound
done
clear
B)
100 pound
done
clear
C)
120 pound
done
clear
D)
150 pound
done
clear
View Answer play_arrow
-
question_answer12) A thin circular ring of mass M and radius r is rotating about its axis with a constant angular velocity \[\omega \]. Two objects each of mass m are attached gently to the opposite ends of diameter of the ring. The ring will now rotate with an angular velocity:
A)
\[\frac{\omega \,(M-2\,m)}{(M+2m)}\]
done
clear
B)
\[\frac{\omega M}{(M+2m)}\]
done
clear
C)
\[\frac{\omega \,M}{(M+m)}\]
done
clear
D)
\[\frac{\omega \,(M+2\,m)}{M}\]
done
clear
View Answer play_arrow
-
question_answer13) A rubber ball is dropped from a height of 5 m on a planet where the acceleration due to gravity is not known. On bouncing it rises to 1.8 m. The ball loses its velocity on bouncing by a factor of:
A)
\[\frac{16}{25}\]
done
clear
B)
\[\frac{2}{5}\]
done
clear
C)
\[\frac{3}{5}\]
done
clear
D)
\[\frac{9}{25}\]
done
clear
View Answer play_arrow
-
question_answer14) Two simple pendulums of length 0.5 m and 2.0 in respectively are given small linear displacement in one direction at die same time. They will again be in the same phase when the pendulum of shorter length has completed oscillations:
A)
5
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
-
question_answer15) A mass m is vertically suspended from a spring of negligible mass; the system oscillates with a frequency n. What will be the frequency of the system, if a mass 4 m is suspended from the same spring ?
A)
\[\frac{n}{4}\]
done
clear
B)
4 n
done
clear
C)
\[\frac{n}{2}\]
done
clear
D)
2 n
done
clear
View Answer play_arrow
-
question_answer16) A particle, with restoring force proportional to displacement and resisting force proportional to velocity is subjected to a force \[F\sin \omega t\]. If the amplitude of the particle is maximum for \[\omega ={{\omega }_{1}}\] and the energy of the particle maximum for \[\omega ={{\omega }_{2}}\], then:
A)
\[\omega ={{\omega }_{0}}\] and \[{{\omega }_{2}}\ne {{\omega }_{0}}\]
done
clear
B)
\[{{\omega }_{1}}={{\omega }_{0}}\] and \[{{\omega }_{2}}={{\omega }_{0}}\]
done
clear
C)
\[{{\omega }_{1}}\ne {{\omega }_{0}}\] and \[{{\omega }_{2}}={{\omega }_{0}}\]
done
clear
D)
\[{{\omega }_{1}}\ne {{\omega }_{0}}\] and \[{{\omega }_{2}}\ne {{\omega }_{0}}\] where \[{{\omega }_{0}}\to \]natural angular frequency of oscillations of particle.
done
clear
View Answer play_arrow
-
question_answer17) If the ratio of specific heat of a gas at constant pressure to that at constant volume is \[\gamma \], the change in internal energy of a mass of gas when the volume changes from V to 2V at constant pressure P is:
A)
\[\frac{R}{(\gamma -1)}\]
done
clear
B)
P V
done
clear
C)
\[\frac{P\,V}{(\gamma -1)}\]
done
clear
D)
\[\frac{\gamma \,PV}{(\gamma -1)}\]
done
clear
View Answer play_arrow
-
question_answer18) We consider a thermodynamic system, If \[\Delta U\] represents the increase in its internal energy and W the work done by the system, which of the following statements is true?
A)
\[\Delta U=-W\] in an adiabatic process
done
clear
B)
\[\Delta U=W\] in an isothermal process
done
clear
C)
\[\Delta U=-W\] in an isothermal process
done
clear
D)
\[\Delta U=W\] in an adiabatic process
done
clear
View Answer play_arrow
-
question_answer19) The radiant energy from the sun, incident normally at the surface of earth is \[20\,kcal/{{m}^{2}}\,\min \]. What would have been the radiant energy, incident normally on the earth, if the sun had a temperature, twice of the present one?
A)
\[160\,kcal/{{m}^{2}}\,\min \]
done
clear
B)
\[40\,kcal/{{m}^{2}}\min \]
done
clear
C)
\[320\,\,kcal/{{m}^{2}}\,\min \]
done
clear
D)
\[80\,\,kcal/{{m}^{2}}\,\min \]
done
clear
View Answer play_arrow
-
question_answer20) A transverse wave is represented by the equation \[y={{y}_{0}}\sin \frac{2\pi }{\lambda }(vt-x)\] For what value of \[\lambda \] is the maximum particle velocity equal to two times the wave velocity?
A)
\[\lambda =2\,\pi {{y}_{0}}\]
done
clear
B)
\[\lambda =\frac{\pi {{v}_{0}}}{3}\]
done
clear
C)
\[\lambda =\frac{\pi {{y}_{0}}}{2}\]
done
clear
D)
\[\lambda =\pi {{y}_{0}}\]
done
clear
View Answer play_arrow
-
question_answer21) A vehicle, with a horn of frequency n is moving with a velocity of 30 m/s in a direction perpendicular to the straight line joining the observer and the vehicle. The observer perceives the sound to have a frequency \[n+{{n}_{1}}\]. Then: (if the sound velocity in air is 300 m/s)
A)
\[{{n}_{1}}=10\,n\]
done
clear
B)
\[{{n}_{1}}=0\]
done
clear
C)
\[{{n}_{1}}=0.1\,n\]
done
clear
D)
\[{{n}_{1}}=-0.1\,n\]
done
clear
View Answer play_arrow
-
question_answer22) In a sinusoidal wave, the time required for a particular point, to move from maximum displacement to zero displacement is 0.170 s. The frequency of the wave is:
A)
1.47 Hz
done
clear
B)
0.36 Hz
done
clear
C)
0.73 Hz
done
clear
D)
2.94 Hz
done
clear
View Answer play_arrow
-
question_answer23) A standing wave having 3 nodes and 2 antinodes is formed between two atoms having a distance \[1.21\,\overset{\text{o}}{\mathop{\text{A}}}\,\] between them. The wavelength of the standing wave is:
A)
\[1.21\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1.42\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[6.05\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[3.63\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
-
question_answer24) A luminous object is placed at a distance of 30 cm from the convex lens of focal length 20 cm. On the other side of the lens, at what distance from the lens, a convex mirror of radius of curvature 10 cm, be placed in order to have an upright image of the object coincident with it?
A)
12 cm
done
clear
B)
30 cm
done
clear
C)
50 cm
done
clear
D)
60 cm
done
clear
View Answer play_arrow
-
question_answer25) Light enters at an angle of incidence in a transparent rod of refractive index n. For what value of the refractive index of the material of the rod the light once entered into it will not leave it through its lateral face whatsoever be the value of angle of incidence?
A)
\[n>\sqrt{2}\]
done
clear
B)
n = 1
done
clear
C)
n = 1.1
done
clear
D)
n = 1.3
done
clear
View Answer play_arrow
-
question_answer26) A point Q lies on die perpendicular bisector of an electrical dipole of dipole moment p. If the distance of Q from the dipole is r (much larger than the size of the dipole) then electric field at Q is proportional to:
A)
\[{{p}^{-1}}\] and \[{{r}^{2}}\]
done
clear
B)
\[p\] and \[{{r}^{-2}}\]
done
clear
C)
\[{{p}^{2}}\] and \[{{r}^{-3}}\]
done
clear
D)
\[p\] and \[{{r}^{-3}}\]
done
clear
View Answer play_arrow
-
question_answer27) A particle of mass m and charge q is placed at rest in a uniform electric field E and then released. The kinetic energy attained by the particle after moving a distance y is:
A)
\[q\,E\,{{y}^{2}}\]
done
clear
B)
\[q\,{{E}^{2}}\,y\]
done
clear
C)
\[q\,E\,y\]
done
clear
D)
\[{{q}^{2}}E\,y\]
done
clear
View Answer play_arrow
-
question_answer28) A hollow insulated conducting sphere is given a positive charge of \[10\,\mu C\]. What will be the electric field at the centre of the sphere if its radius is 2 m ?
A)
Zero
done
clear
B)
\[5\,\mu \,C{{m}^{-2}}\]
done
clear
C)
\[20\,\mu \,C{{m}^{-2}}\]
done
clear
D)
\[8\,\mu \,C{{m}^{-2}}\]
done
clear
View Answer play_arrow
-
question_answer29) Three equal resistors connected in series across .a source of emf together dissipate 10 watt of power. What will be the power dissipated in watt if the same resistors are connected in parallel across the same source of emf?
A)
10/3
done
clear
B)
10
done
clear
C)
30
done
clear
D)
90
done
clear
View Answer play_arrow
-
question_answer30) A \[{{5}^{\text{o}}}C\] rise in temperature is observed in a conductor by passing a current. When the current is doubled the rise in temperature will be approximately:
A)
\[{{16}^{\text{o}}}C\]
done
clear
B)
\[{{10}^{\text{o}}}C\]
done
clear
C)
\[{{20}^{\text{o}}}C\]
done
clear
D)
\[{{12}^{\text{o}}}C\]
done
clear
View Answer play_arrow
-
question_answer31) If nearly \[{{10}^{5}}C\] liberate 1 g equivalent of aluminium, then the amount of aluminium (equivalent weight 9) deposited through electrolysis in 20 min by a current of 50 amp will be:
A)
0.6 g
done
clear
B)
0.09 g
done
clear
C)
5.4 g
done
clear
D)
10.8 g
done
clear
View Answer play_arrow
-
question_answer32) A galvanometer having a resistance of 8 ohm is shunted by a wire of resistance 2 ohm. If the total current is 1 A, the part of it passing through the shunt will be:
A)
0.25 A
done
clear
B)
0.8 A
done
clear
C)
0.2 A
done
clear
D)
0.5 A
done
clear
View Answer play_arrow
-
question_answer33) A coil of one turn is made of a wire of certain length and then from the same length a coil of two turns is made. If the same current is passed in both the cases, then the ratio of the magnetic induction at their centres will be:
A)
2 : 1
done
clear
B)
1 : 4
done
clear
C)
4 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
-
question_answer34) Two long parallel wires are at a distance of 1 m. Both of them carry one ampere of current. The force of attraction per unit length between the two wires is:
A)
\[2\times {{10}^{-7}}\,N/m\]
done
clear
B)
\[2\times {{10}^{-8}}\,N/m\]
done
clear
C)
\[5\times {{10}^{-8}}\,N/m\]
done
clear
D)
\[{{10}^{-7}}\,N/m\]
done
clear
View Answer play_arrow
-
question_answer35) For protecting a sensitive equipment from the external magnetic field, it should be:
A)
placed inside an aluminium can
done
clear
B)
placed inside an iron can
done
clear
C)
wrapped with insulation around it when passing current through it
done
clear
D)
surrounded with fine copper sheet
done
clear
View Answer play_arrow
-
question_answer36) Two coils have a mutual inductance of 0.005 H. The current changes in the first coil according to equation \[I={{I}_{0}}\,\sin \omega t\], where \[{{I}_{0}}=10\,A\] and \[\omega =100\,\pi \] rad/s. The maximum value of emf in the second coil is:
A)
\[2\,\pi \]
done
clear
B)
\[5\,\pi \]
done
clear
C)
\[\,\pi \]
done
clear
D)
\[4\pi \]
done
clear
View Answer play_arrow
-
question_answer37) A step-up transformer operates on a 230 V line and supplies current of 2A to a load. The ratio of the primary and secondary windings is 1 : 25. The current in the primary coil is:
A)
15 A
done
clear
B)
50 A
done
clear
C)
25 A
done
clear
D)
12.5 A
done
clear
View Answer play_arrow
-
question_answer38) The 21 cm radio wave emitted by hydrogen in interstellar space is due to the interaction called the hyperfine interaction in atomic hydrogen. The energy of the emitted wave is nearly:
A)
\[{{10}^{-17}}\,J\]
done
clear
B)
1 J
done
clear
C)
\[7\times {{10}^{-6}}\,J\]
done
clear
D)
\[{{10}^{-24}}\,J\]
done
clear
View Answer play_arrow
-
question_answer39) In the Bohr's model of a hydrogen atom, the centripetal force is furnished by the Coulomb attraction between the proton and the electron. If \[{{a}_{0}}\] is the radius of the ground state orbit, m is the mass and e is the charge on the electron, \[{{\varepsilon }_{0}}\] is the vacuum permittivity, the speed of the electron is:
A)
zero
done
clear
B)
\[\frac{e}{\sqrt{{{\varepsilon }_{0}}{{a}_{0}}m}}\]
done
clear
C)
\[\frac{e}{\sqrt{4\pi {{\varepsilon }_{0}}{{a}_{0}}m}}\]
done
clear
D)
\[\frac{\sqrt{4\pi {{\varepsilon }_{0}}{{a}_{0}}m}}{e}\]
done
clear
View Answer play_arrow
-
question_answer40) Light of wavelength \[5000\overset{\text{o}}{\mathop{\text{A}}}\,\] falls on a sensitive plate with photoelectric work function of 1.9 eV. The kinetic energy of the photoelectron emitted will be:
A)
0.58 eV
done
clear
B)
2.48 eV
done
clear
C)
1.24 eV
done
clear
D)
1.16 eV
done
clear
View Answer play_arrow
-
question_answer41) In a photo-emissive cell, with exciting wavelength \[\lambda \], the fastest electron has speed v. If the exciting wavelength is changed to \[3\lambda /4\], the speed of the fastest emitted electron will be:
A)
\[v\,{{(3/4)}^{1/2}}\]
done
clear
B)
\[v{{(4/3)}^{1/2}}\]
done
clear
C)
less than \[v{{(4/3)}^{1/2}}\]
done
clear
D)
greater than \[v{{(4/3)}^{1/2}}\]
done
clear
View Answer play_arrow
-
question_answer42) Half-lives of two radioactive substances A and B are respectively 20 min and 40 min. Initially the samples of A and B have equal number of nuclei. After 80 min the ratio of remaining number of A and B nuclei is:
A)
1 : 16
done
clear
B)
4 : 1
done
clear
C)
1 : 4
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
-
question_answer43) Atomic weight of boron is 10.81 and it has two isotopes \[_{5}^{10}B\] and \[_{5}^{11}B.\] Then, the ratio of atoms of \[_{5}^{10}B\] and \[_{5}^{11}B\] in nature, would be:
A)
19 : 81 Let \[{{n}_{1}}\] and \[{{n}_{2}}\] be the number of atoms in \[_{5}^{10}B\] and \[_{5}^{11}B\] isotopes. Atomic weight \[=\frac{{{n}_{1}}\times (At.\,wt.\,of\,_{5}^{10}B)+{{n}_{2}}\times (At.wt.\,of\,_{5}^{11}B)}{{{n}_{1}}+{{n}_{2}}}\] \[or10.81=\frac{{{n}_{1}}\times 10+{{n}_{2}}\times 11}{{{n}_{1}}+{{n}_{2}}}\] \[or10.81\,{{n}_{1}}+10.81\,{{n}_{2}}=10\,{{n}_{1}}+11\,{{n}_{2}}\] \[or0.81\,\,{{n}_{1}}=0.19\,{{n}_{2}}\] \[or\frac{{{n}_{1}}}{{{n}_{2}}}=\frac{0.19}{0.81}=\frac{19}{81}\]
done
clear
B)
10 : 11
done
clear
C)
15 : 16
done
clear
D)
81 : 19
done
clear
View Answer play_arrow
-
question_answer44) A nucleus \[_{n}{{X}^{m}}\] emits one \[\alpha \] and two \[\beta \] particles The resulting nucleus is:
A)
\[_{n}{{X}^{m-4}}\]
done
clear
B)
\[_{n-2}{{Y}^{m-4}}\]
done
clear
C)
\[_{n-4}{{Z}^{m-4}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
-
question_answer45) Complete the equation for the following fission process: \[_{92}{{U}^{235}}+{{\,}_{0}}{{n}^{1}}\,\xrightarrow[{}]{{}}{{\,}_{38}}S{{r}^{90}}+.......\]
A)
\[_{54}X{{e}^{143}}+\,3{{\,}_{0}}{{n}^{1}}\]
done
clear
B)
\[_{54}X{{e}^{145}}\]
done
clear
C)
\[_{57}X{{e}^{142}}\]
done
clear
D)
\[_{54}X{{e}^{142}}+{{\,}_{0}}{{n}^{1}}\]
done
clear
View Answer play_arrow
-
question_answer46) The cause of the potential barrier in a p-n diode is:
A)
depletion of positive charges near the junction
done
clear
B)
concentration of positive charges near the junction
done
clear
C)
depletion of negative charges near the junction
done
clear
D)
concentration of positive and negative charges near the junction
done
clear
View Answer play_arrow
-
question_answer47) A semi-conducting device is connected in a series in circuit with a battery and a resistance. A current is allowed to pass through the circuit. If the polarity of the battery is reversed, the current drops to almost zero. The device may be:
A)
a p-n junction
done
clear
B)
an intrinsic semiconductor
done
clear
C)
a p-type semiconductor
done
clear
D)
an n-type semiconductor
done
clear
View Answer play_arrow
-
question_answer48) The transfer ratio \[\beta \] of a transistor is 50. The input resistance of the transistor when used in the common emitter configuration is \[1\,k\,\Omega \]. The peak value of the collector AC current for an AC input voltage of 0.01 V peak is:
A)
\[100\,\mu A\]
done
clear
B)
\[0.01\,mA\]
done
clear
C)
\[0.25\,mA\]
done
clear
D)
\[500\,\mu A\]
done
clear
View Answer play_arrow
-
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
View Answer play_arrow
-
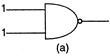
question_answer50)
The truth table given below is for which gate? | Input | Output |
| A | B | C |
| 0 | 0 | 1 |
| 0 | 1 | 1 |
| 1 | 0 | 1 |
| 1 | 1 | 0 |
A)
XOR
done
clear
B)
OR
done
clear
C)
AND
done
clear
D)
NAND
done
clear
View Answer play_arrow
-
question_answer51) Given the number, 161 cm, 0.161 cm, 0.0161 cm. The number of significant figures for the three numbers is:
A)
3, 4 and 5, respectively
done
clear
B)
3, 4 and 4, respectively
done
clear
C)
3, 3 and 4, respectively
done
clear
D)
3, 3 and 3, respectively
done
clear
View Answer play_arrow
-
question_answer52) Haemoglobin contains 0.33% of iron by weight. The molecular weight of haemoglobin is approximately 67200. The number of iron atoms (at. wt. of Fe is 56) present in one molecule of haemoglobin are:
A)
1
done
clear
B)
6
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
-
question_answer53) Bohr radius for the hydrogen atom (n = 1) is approximately 0.530 \[\overset{\text{o}}{\mathop{\text{A}}}\,\]. The radius for the first excited state (n = 2) orbit is (in \[\overset{\text{o}}{\mathop{\text{A}}}\,\]):
A)
0.13
done
clear
B)
1.06
done
clear
C)
4.77
done
clear
D)
2.12
done
clear
View Answer play_arrow
-
question_answer54) The position of both, an electron and a helium atom is known within 1.0 mm. Further the momentum of the election is known within \[\text{5}\text{.0}\times \text{1}{{\text{0}}^{-26}}\,kg\,m{{s}^{-1}}\]. The minimum uncertainty in the measurement of the momentum of the helium atom is:
A)
\[50\,kg\,m{{s}^{-1}}\]
done
clear
B)
\[80\,kg\,m{{s}^{-1}}\]
done
clear
C)
\[80\times {{10}^{-26}}\,kg\,m{{s}^{-1}}\]
done
clear
D)
\[5.0\times {{10}^{-26}}\,kg\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer55) Which one is not paramagnetic among the following?
A)
[Atomic number: Be = 4, Ne = 10, As = 33, CI = 17] \[C{{l}^{-}}\]
done
clear
B)
Be
done
clear
C)
\[N{{e}^{2+}}\]
done
clear
D)
\[A{{s}^{+}}\]
done
clear
View Answer play_arrow
-
question_answer56) Number of neutrons in a parent nucleus X, which gives \[_{7}{{N}^{14}}\] nucleus after two successive \[\beta \]-emmissions would be:
A)
9
done
clear
B)
6
done
clear
C)
7
done
clear
D)
8
done
clear
View Answer play_arrow
-
question_answer57) In \[PO_{4}^{3-}\] ion, the formal charge on each oxygen atom and P?O bond order respectively are:
A)
- 0.75, 0.6
done
clear
B)
- 0.75, 1.0
done
clear
C)
- 0.75, 1.25
done
clear
D)
- 3, 1.25
done
clear
View Answer play_arrow
-
question_answer58) The number of anti-bonding electrons in \[O_{2}^{2-}\] molecular ion on the basis of molecular orbital theory is (Atomic no. of O is 8):
A)
5
done
clear
B)
2
done
clear
C)
4
done
clear
D)
6
done
clear
View Answer play_arrow
-
question_answer59) Schottky defect in crystals is observed when:
A)
an ion leaves its normal site and occupies an interstitial site
done
clear
B)
unequal number of cations and anions are missing from the lattice
done
clear
C)
density of the crystal is increased
done
clear
D)
equal number of cations and anions are missing from the lattice
done
clear
View Answer play_arrow
-
question_answer60) The edge length of face centred unit cubic cell is 508 pm. If the radius of the cation is 110 pm, the radius of the anion is:
A)
288 pm
done
clear
B)
398 pm
done
clear
C)
144 pm
done
clear
D)
618 pm
done
clear
View Answer play_arrow
-
question_answer61) The second order Bragg diffraction of X-rays with \[\lambda =1.0\,\overset{\text{o}}{\mathop{\text{A}}}\,\] from a set of parallel planes in a metal occurs at an angle 60°. The distance between the scattering planes in the crystals is:
A)
\[0.575\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1.00\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[2.00\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1.17\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
-
question_answer62) In crystals of which of the following ionic compounds would you expect maximum distance between centres of cations and anions?
A)
LiF
done
clear
B)
CsF
done
clear
C)
Csl
done
clear
D)
Lil
done
clear
View Answer play_arrow
-
question_answer63) An organic compound containing C, H and N gave the following results on analysis C = 40%, H = 13.33%, N = 46.67%. Its empirical formula would be:
A)
\[{{C}_{2}}{{H}_{7}}\,{{N}_{2}}\]
done
clear
B)
\[C{{H}_{5}}N\]
done
clear
C)
\[C{{H}_{4}}N\]
done
clear
D)
\[{{C}_{2}}{{H}_{7}}N\]
done
clear
View Answer play_arrow
-
question_answer64) A 5% solution of cane sugar (mol. wt. = 342) is isotonic with 1% solution of a substance X. The molecular weight of X is:
A)
34.2
done
clear
B)
171.2
done
clear
C)
68.4
done
clear
D)
136.8
done
clear
View Answer play_arrow
-
question_answer65) The vapour pressure of a solvent decreased by 10 mm in two columns of mercury when a non-volatile solute was added to the solvent. The mole fraction of the solute in the solution is 0.2. What should be the mole fraction of the solvent if the decrease in the vapour pressure is to be 20 mm of mercury?
A)
0.8
done
clear
B)
0.6
done
clear
C)
0.4
done
clear
D)
0.2
done
clear
View Answer play_arrow
-
question_answer66) If \[{{K}_{1}}\] and \[{{K}_{2}}\] are the respective equilibrium constants for the two reactions: \[Xe{{F}_{6}}\left( g \right)+{{H}_{2}}O\left( g \right)~\rightleftharpoons XeO{{F}_{4}}\left( g \right)+2HF\left( g \right)\] \[Xe{{O}_{4}}\left( g \right)+Xe{{F}_{6}}\left( g \right)~\rightleftharpoons XeO{{F}_{4}}\left( g \right)+Xe{{O}_{3}}{{F}_{2}}\left( g \right)\] the equilibrium constant of the reaction \[Xe{{O}_{4}}\left( g \right)+2HF\left( g \right)\rightleftharpoons ~Xe{{O}_{3}}{{F}_{2}}\left( g \right)+{{H}_{2}}O\left( g \right)\] will be:
A)
\[{{K}_{2}}/{{({{K}_{2}})}^{2}}\]
done
clear
B)
\[{{K}_{1}}\,.\,{{K}_{2}}\]
done
clear
C)
\[{{K}_{1}}/{{K}_{2}}\]
done
clear
D)
\[{{K}_{2}}/{{K}_{1}}\]
done
clear
View Answer play_arrow
-
question_answer67) In the reaction \[4N{{H}_{3}}(g)+5{{O}_{2}}(g)\to 4NO(g)\,+6{{H}_{2}}O(l)\] when 1 mole of ammonia and 1 mole of \[{{O}_{2}}\] are made to react to completion then :
A)
1.0 mole of \[{{H}_{2}}O\] is produced
done
clear
B)
1.0 mole of NO will be produced
done
clear
C)
all the oxygen will be consumed
done
clear
D)
all the ammonia will be consumed
done
clear
View Answer play_arrow
-
question_answer68) Identify the correct statement regarding entropy:
A)
At absolute zero temperature, entropy of a perfectly crystalline substance is taken to be zero
done
clear
B)
At absolute zero of temperature the entropy of a perfectly crystalline substance is +ve
done
clear
C)
At absolute zero of temperature the entropy of all crystalline substance is to be zero
done
clear
D)
At \[{{0}^{\text{o}}}C,\] the entropy of a perfectly crystalline substance is taken to be zero
done
clear
View Answer play_arrow
-
question_answer69) Activation energy of a chemical reaction can be determined by:
A)
evaluating rate constant at standard temperature
done
clear
B)
evaluating velocities of reaction at two different temperatures
done
clear
C)
evaluating rate constants at two different temperatures
done
clear
D)
changing concentration of reactants
done
clear
View Answer play_arrow
-
question_answer70) One mole of an ideal gas at 300K is expanded isothermally from an initial volume of 1 L to 10L. The \[\Delta E\]for this process is \[(R=2\,cal\,mo{{l}^{-1}}{{K}^{-1}})\]
A)
163.7 cal
done
clear
B)
zero
done
clear
C)
1381.1 cal
done
clear
D)
9 L atm
done
clear
View Answer play_arrow
-
question_answer71) For the cell reaction, \[C{{u}^{2+}}({{C}_{1}}\,aq)+Zn(s)\rightleftharpoons Z{{n}^{2+}}({{C}_{2}}\,aq)+Cu(s)\] of an electrochemical cell. The change in free energy \[(\Delta G)\] at a given temperature is a function of:
A)
\[\ln \,({{C}_{1}})\]
done
clear
B)
\[\ln \,({{C}_{2}}/{{C}_{1}})\]
done
clear
C)
\[\ln \,({{C}_{2}})\]
done
clear
D)
\[\ln \,({{C}_{1}}+{{C}_{2}})\]
done
clear
View Answer play_arrow
-
question_answer72) Without losing its concentration \[ZnC{{l}_{2}}\] solution cannot be kept in contact with:
A)
Au
done
clear
B)
Al
done
clear
C)
Pb
done
clear
D)
Ag
done
clear
View Answer play_arrow
-
question_answer73) At the critical micelle concentration (cmc) the surfactant molecules:
A)
decompose
done
clear
B)
dissociate
done
clear
C)
associate
done
clear
D)
become completely soluble
done
clear
View Answer play_arrow
-
question_answer74)
IUPAC name of the compounds 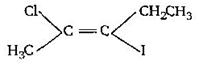
A)
trans-3 iodo- 4 chloro-3-pentene
done
clear
B)
cis-2-chloro-3-iodo-2-pentene
done
clear
C)
trans-2-chloro-3-iodo-2-pentene
done
clear
D)
cis-3-iodo-4-chloro-3-pentene
done
clear
View Answer play_arrow
-
question_answer75) Which of the following compounds is not chiral?
A)
\[DC{{H}_{2}}C{{H}_{2}}C{{H}_{2}}Cl\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CHDCl\]
done
clear
C)
\[C{{H}_{3}}CHDC{{H}_{2}}Cl\]
done
clear
D)
\[C{{H}_{3}}CHClC{{H}_{2}}D\]
done
clear
View Answer play_arrow
-
question_answer76) Which one of the following orders is correct regarding the \[-I\] effect of the substituents?
A)
\[-N{{R}_{2}}<-OR>-F\]
done
clear
B)
\[-N{{R}_{2}}>-OR>-F\]
done
clear
C)
\[-N{{R}_{2}}<-OR<-F\]
done
clear
D)
\[-N{{R}_{2}}>-OR<-F\]
done
clear
View Answer play_arrow
-
question_answer77) Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?
A)
Methyl acetate
done
clear
B)
Acetonitrile
done
clear
C)
Dimethyl ether
done
clear
D)
Acetamide
done
clear
View Answer play_arrow
-
question_answer78) 2-bromopentane is heated with potassium ethoxide in ethanol. The major product obtained is:
A)
2-ethoxypentane
done
clear
B)
pentene-1
done
clear
C)
trans-pentene-2
done
clear
D)
cis-pentene-2
done
clear
View Answer play_arrow
-
question_answer79)
Reaction of 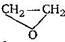 with \[RMgX\] leads to formation of:
with \[RMgX\] leads to formation of:
A)
RCHOHR
done
clear
B)
\[RCHOHC{{H}_{3}}\]
done
clear
C)
\[RC{{H}_{2}}C{{H}_{2}}OH\]
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer80) Glucose molecule reacts with 'X' number of molecules of phenylhydrazine to yield osazone. The value of ?X? is:
A)
four
done
clear
B)
one
done
clear
C)
two
done
clear
D)
three
done
clear
View Answer play_arrow
-
question_answer81) Iodoform test is not given by:
A)
2-pentanone
done
clear
B)
ethanol
done
clear
C)
ethanal
done
clear
D)
3-pentanone
done
clear
View Answer play_arrow
-
question_answer82) An ester (A) with molecular formula \[{{C}_{9}}{{H}_{10}}{{O}_{2}}\] was treated with excess of \[C{{H}_{3}}MgBr\] and the complex so formed was treated with \[{{H}_{2}}S{{O}_{4}}\] to give an olefin (B). Ozonolysis of (B) gave a ketone with molecular formula \[{{C}_{8}}{{H}_{8}}O\] which shows +ve iodoform test. The structure of (A) is:
A)
\[{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}COO{{C}_{6}}{{H}_{5}}\]
done
clear
C)
\[{{H}_{3}}COC{{H}_{2}}CO{{C}_{6}}{{H}_{5}}\]
done
clear
D)
\[p-{{H}_{3}}CO-{{C}_{6}}{{H}_{4}}-COC{{H}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer83) Which one of the following esters cannot undergo Claisen self-condensation?
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{11}}C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
-
question_answer84) Which one of these, is not compatible with arenes?
A)
Greater stability
done
clear
B)
Delocalisation of \[\pi \]-electrons
done
clear
C)
Electrophilic additions
done
clear
D)
Resonance
done
clear
View Answer play_arrow
-
question_answer85) Which one of the following compounds will be most easily attached by an electrophile?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer86) Aniline is reacted with bromine water and the resulting product is treated with an aqueous solution of sodium nitrite in presence of dilute hydrochloric acid. The compound so formed is converted to a tetrafluoroborate which is subsequently heated. The final product is:
A)
1, 3, 5-tribromobenzene
done
clear
B)
p-bromofluorobenzene
done
clear
C)
p-bromoaniline
done
clear
D)
2, 4, 6-tribromofluorobenzene
done
clear
View Answer play_arrow
-
question_answer87) Aspirin is an acetylation product of:
A)
o-hydroxybenzoic acid
done
clear
B)
o-hydroxybenzene
done
clear
C)
m-hydroxybenzoic acid
done
clear
D)
p-dihydroxybenzene
done
clear
View Answer play_arrow
-
question_answer88) The number of molecules of ATP produced in the lipid metabolism of a molecule of palmitic acid is:
A)
130
done
clear
B)
36
done
clear
C)
56
done
clear
D)
86
done
clear
View Answer play_arrow
-
question_answer89) In DNA the complementary bases are:
A)
adenine and thymine, guanine and cytosine
done
clear
B)
uracil and adenine, cytosine and guanine
done
clear
C)
adenine and guanine, thymine and cytosine
done
clear
D)
adenine and thymine, guanine and uracil
done
clear
View Answer play_arrow
-
question_answer90) The first ionization potential (in eV) of Be and B, respectively are:
A)
8.29, 9.32
done
clear
B)
9.32, 9.32
done
clear
C)
8.29, 8.29
done
clear
D)
9.32, 8.29
done
clear
View Answer play_arrow
-
question_answer91) The total number of possible isomers for the complex compound \[[C{{u}^{II}}{{(N{{H}_{3}})}_{4}}][P{{t}^{II}}C{{l}_{4}}]\]
A)
3
done
clear
B)
6
done
clear
C)
5
done
clear
D)
4
done
clear
View Answer play_arrow
-
question_answer92) IUPAC name of \[[Pt{{(N{{H}_{3}})}_{3}}\,(Br)\,(N{{O}_{2}})\,Cl]\,Cl\] is:
A)
Triammine chlorobromonitro platinum (IV) chloride
done
clear
B)
Triammine bromonitrochloro platinum (IV) chloride
done
clear
C)
Triammine bromochloronitro platinum (IV) chloride
done
clear
D)
Triammine nitrochlorobromo platinum (IV) chloride
done
clear
View Answer play_arrow
-
question_answer93) A co-ordination complex compound of cobalt has the molecular formula containing five ammonia molecules, one nitro group and two chlorine atoms for one cobalt atom. One mole of this compound produces three mole ions in an aqueous solution. On reacting this solution with excess of \[AgN{{O}_{3}}\] solution, we get two moles of \[AgCl\] precipitate. The ionic formula for this complex would be:
A)
\[[Co{{(N{{H}_{3}})}_{4}}(N{{O}_{2}})Cl]\,[(N{{H}_{3}}Cl]\]
done
clear
B)
\[[Co\,(N{{H}_{3}})Cl]\,[Cl(N{{O}_{2}})]\]
done
clear
C)
\[[Co{{(N{{H}_{3}})}_{5}}(N{{O}_{2}})]C{{l}_{2}}\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{5}}]\,[{{(N{{O}_{2}})}_{2}}C{{l}_{2}}]\]
done
clear
View Answer play_arrow
-
question_answer94) Which one of the following elements shows maximum number of different oxidation states in its compounds?
A)
Eu
done
clear
B)
La
done
clear
C)
Gd
done
clear
D)
Am
done
clear
View Answer play_arrow
-
question_answer95) Which one of the following elements constitutes a major impurity in pig iron?
A)
Silicon
done
clear
B)
Oxygen
done
clear
C)
Sulphur
done
clear
D)
Graphite
done
clear
View Answer play_arrow
-
question_answer96) When a substance A reacts with water it produces a combustible gas B and a solution of substance C in water. When another substance D reacts with this solution of C, it also produces the same gas B on warming but D can produce gas B on reaction with dilute sulphuric acid at room temperature. ?A? imparts a deep golden yellow colour to a smokeless flame of bunsen burner A, B, C and D respectively are:
A)
\[Na,\,{{H}_{2}},\,NaOH,\,Zn\]
done
clear
B)
\[K,\,{{H}_{2}},\,KOH,\,Al\]
done
clear
C)
\[Ca{{H}_{2}},\,Ca{{(OH)}_{2}},\,Sn\]
done
clear
D)
\[Ca{{C}_{2}},\,{{C}_{2}}{{H}_{2}},\,Ca{{(OH)}_{2}},\,Fe\]
done
clear
View Answer play_arrow
-
question_answer97) Which one of the following pairs of substances on reaction will not evolve \[{{H}_{2}}\] gas?
A)
Iron and \[{{H}_{2}}S{{O}_{4}}\] (aqueous)
done
clear
B)
Iron and steam
done
clear
C)
Copper and HCl (aqueous)
done
clear
D)
Sodium and ethyl alcohol
done
clear
View Answer play_arrow
-
question_answer98) A one litre flask is full of brown bromine vapour. The intensity of brown colour of vapour will not decrease appreciably on adding to the flask some:
A)
pieces of marble
done
clear
B)
animal charcoal powder
done
clear
C)
carbon tetrachloride
done
clear
D)
carbon disulphide
done
clear
View Answer play_arrow
-
question_answer99) Repeated use of which one of the following fertilizers would increase the acidity of the soil?
A)
Urea
done
clear
B)
Superphosphate of lime
done
clear
C)
Ammonium sulphate
done
clear
D)
Potassium nitrate
done
clear
View Answer play_arrow
-
question_answer100) Which one of the following ionic species will impart colour to an aqueous solution?
A)
\[T{{i}^{4+}}\]
done
clear
B)
\[C{{u}^{+}}\]
done
clear
C)
\[Z{{n}^{2+}}\]
done
clear
D)
\[C{{r}^{3+}}\]
done
clear
View Answer play_arrow
-
question_answer101) The reason why vagetatively reproducing crop plants are best suited for maintaining hybrid vigour is that:
A)
they can be easily propagated
done
clear
B)
they have a longer life span
done
clear
C)
they are more resistant to dieases
done
clear
D)
once a desired hybrid is produced, there are no chances of losing it
done
clear
View Answer play_arrow
-
question_answer102) An adult human with average health has systolic and diastolic pressures as:
A)
80 mm Hg and 80 mm Hg
done
clear
B)
70 mm Hg and 120 mm Hg
done
clear
C)
120 mm Hg and 80 mm Hg
done
clear
D)
50 mm Hg and 80 mm Hg
done
clear
View Answer play_arrow
-
question_answer103) The formation of multivalents at meiosis in diploid organism is due to
A)
monosomy
done
clear
B)
inversion
done
clear
C)
deletion
done
clear
D)
reciprocal translocation
done
clear
View Answer play_arrow
-
question_answer104) In the developmental history of mammalian heart, it is observed that it passes through a two-chambered fish-like heart, three chambered frog-like heart and finally four-chambered stage. To which hypothesis can this above cited statement be approximated?
A)
Biogenetic law
done
clear
B)
Hardy-Weinberg law
done
clear
C)
Lamarck's principle
done
clear
D)
Mendelian principles
done
clear
View Answer play_arrow
-
question_answer105) Which of the following is the contractile protein of a muscle?
A)
Tubulin
done
clear
B)
Myosin
done
clear
C)
Tropomyosin
done
clear
D)
All of these
done
clear
View Answer play_arrow
-
question_answer106) Which of the micro-organisms is used for production of citric acid in industries?
A)
Lactobacillus bulgaris
done
clear
B)
Pencillium citrinum
done
clear
C)
Aspergillus niger
done
clear
D)
Rhizopus nigricans
done
clear
View Answer play_arrow
-
question_answer107) Which of the following is not main function of lymph gland?
A)
Forming WBC
done
clear
B)
Forming antibodies
done
clear
C)
Forming RBC
done
clear
D)
Destroying bacteria
done
clear
View Answer play_arrow
-
question_answer108) In mammals, histamine is secreted by:
A)
fibroblasts
done
clear
B)
histocytes
done
clear
C)
lymphocytes
done
clear
D)
mast cells
done
clear
View Answer play_arrow
-
question_answer109) Which one of the following organisms is used as indicator of water quality?
A)
Beggiatoa
done
clear
B)
Chlorella
done
clear
C)
Azospirilhan
done
clear
D)
Escherichia
done
clear
View Answer play_arrow
-
question_answer110) Transfusion tissue is present in the leaves of:
A)
Dryopteris
done
clear
B)
Cycas
done
clear
C)
Pinus
done
clear
D)
Both (b) and (c)
done
clear
View Answer play_arrow
-
question_answer111) The hormone that stimulates the stomach to secrete gastric juice is:
A)
gastrin
done
clear
B)
rennin
done
clear
C)
enterokinase
done
clear
D)
enterogastrone
done
clear
View Answer play_arrow
-
question_answer112) Photochrome becomes active in:
A)
green light
done
clear
B)
blue light
done
clear
C)
red light
done
clear
D)
none of these
done
clear
View Answer play_arrow
-
question_answer113) Which one of the following statements about Cycas is incorrect?
A)
It roots contain some blue-green algae
done
clear
B)
It does not have a well organized female flower
done
clear
C)
It has circinate vernation
done
clear
D)
Its xylem is mainly composed of xylem vessel
done
clear
View Answer play_arrow
-
question_answer114) Plants such as Prosopis, Acacia and Capparis represent examples of tropical:
A)
grass lands
done
clear
B)
thorn forests
done
clear
C)
deciduous forests
done
clear
D)
evergreen forests
done
clear
View Answer play_arrow
-
question_answer115) The rate at which light energy is converted into chemical energy of organic molecules is the ecosystem's:
A)
net primary productivity
done
clear
B)
gross secondary productivity
done
clear
C)
net secondary productivity
done
clear
D)
gross primary productivity
done
clear
View Answer play_arrow
-
question_answer116) The layer of cells that secrete enamel of tooth is:
A)
dentoblast
done
clear
B)
ameloblast
done
clear
C)
osteoblast
done
clear
D)
odontoblast
done
clear
View Answer play_arrow
-
question_answer117) Which one among the following chemicals is used for causing defoliation of forest trees?
A)
Amo-1618
done
clear
B)
Phosphon-D
done
clear
C)
Malic hydrazide
done
clear
D)
2, 4-D
done
clear
View Answer play_arrow
-
question_answer118) Two opposite forces operate in the growth and development of every population. One of them is related to the ability to reproduce at a given rate. The force opposing to it is called:
A)
biotic control
done
clear
B)
mortality
done
clear
C)
fecundity
done
clear
D)
environmental resistance
done
clear
View Answer play_arrow
-
question_answer119) One of the factors required for the maturation of erythrocytes is:
A)
vitamin D
done
clear
B)
vitamin A
done
clear
C)
vitamin \[{{B}_{12}}\]
done
clear
D)
vitamin C
done
clear
View Answer play_arrow
-
question_answer120) The hormone which regulates the basal metabolism in our body is secreted from:
A)
pituitary
done
clear
B)
thyroid
done
clear
C)
adrenal cortex
done
clear
D)
Pancreas
done
clear
View Answer play_arrow
-
question_answer121) Loss of an X-chromosoroe in a particular cell, during its development, results into:
A)
diploid individual
done
clear
B)
triploid individual
done
clear
C)
gynandromorphs
done
clear
D)
both 'a' and 'b?
done
clear
View Answer play_arrow
-
question_answer122) Yeast (Saccharomyces cerevisiae) is used the industrial production of:
A)
butanal
done
clear
B)
citric acid
done
clear
C)
tetracycline
done
clear
D)
ethanol
done
clear
View Answer play_arrow
-
question_answer123) Which important green-house gas, other than \[C{{O}_{2}}\], is being produced from the agricultural fields?
A)
Arsine
done
clear
B)
Sulphur dioxide
done
clear
C)
Ammonia
done
clear
D)
Nitrous oxide
done
clear
View Answer play_arrow
-
question_answer124) Carbon mono-oxide is a pollutant because:
A)
it reacts with \[{{O}_{2}}\]
done
clear
B)
it inhibits glycolysis
done
clear
C)
reacts with haemoglobin
done
clear
D)
makes nervous system inactive
done
clear
View Answer play_arrow
-
question_answer125) A plant hormone used for inducing morphogenesis in plant tissue culture is:
A)
abscisic acid
done
clear
B)
gibberellins
done
clear
C)
cytokinins
done
clear
D)
Ethylene
done
clear
View Answer play_arrow
-
question_answer126) The exchange of gases in the alveoli of the lungs takes place by:
A)
osmosis
done
clear
B)
simple diffusion
done
clear
C)
passive transport
done
clear
D)
active transport
done
clear
View Answer play_arrow
-
question_answer127) If there was no \[C{{O}_{2}}\] in the earth's atmosphere the temperature of earth's surface would be:
A)
same as present
done
clear
B)
less than the present
done
clear
C)
higher than the present
done
clear
D)
dependent on the amount of oxygen in the atmosphere
done
clear
View Answer play_arrow
-
question_answer128) When a single gene influences more than one traits it is called:
A)
pleiocropy
done
clear
B)
epistasis
done
clear
C)
pseudodominance
done
clear
D)
none of these
done
clear
View Answer play_arrow
-
question_answer129) The role of double fertilization in angiosperms is to produce:
A)
endosperm
done
clear
B)
integuments
done
clear
C)
cotyledons
done
clear
D)
Endocarp
done
clear
View Answer play_arrow
-
question_answer130) Total number of bones in the hind limb of a man is:
A)
14
done
clear
B)
21
done
clear
C)
24
done
clear
D)
30
done
clear
View Answer play_arrow
-
question_answer131) If Mendel had studied the seven traits using a plant with 12 chromosomes instead of 14, in what way would his interpretation have been different?
A)
He would have mapped the chromosome
done
clear
B)
He would have discovered blending or incomplete dominance
done
clear
C)
He would not have discovered the law of independent assortment
done
clear
D)
He would have discovered sex linkage
done
clear
View Answer play_arrow
-
question_answer132) The lower jaw in mammals is made up of:
A)
angulars
done
clear
B)
mandible
done
clear
C)
dentary
done
clear
D)
Maxills
done
clear
View Answer play_arrow
-
question_answer133) Botulism caused by Clostridium botulinum affects the:
A)
spleen
done
clear
B)
intestine
done
clear
C)
lymph glands
done
clear
D)
neuromuscular junction
done
clear
View Answer play_arrow
-
question_answer134) Which of the following is non-symbiotic biofcrtilizer?
A)
VAM
done
clear
B)
Azotobacter
done
clear
C)
Anabaena
done
clear
D)
Rhizobium
done
clear
View Answer play_arrow
-
question_answer135) The most important component of die oral contraceptive pills is:
A)
progesterone
done
clear
B)
growth hormone
done
clear
C)
thyroxine
done
clear
D)
luteinzing hormone
done
clear
View Answer play_arrow
-
question_answer136) The contraction of gall bladder is due to:
A)
gastrin
done
clear
B)
secretin
done
clear
C)
cholecystokinin
done
clear
D)
enterogastrone
done
clear
View Answer play_arrow
-
question_answer137) Microtubule is involved in the:
A)
cell division
done
clear
B)
DNA recognition
done
clear
C)
muscle contraction
done
clear
D)
membrane architecture
done
clear
View Answer play_arrow
-
question_answer138) Which base is responsible for hot spots for spontaneous point mutations?
A)
Guanine
done
clear
B)
Adenine
done
clear
C)
5-bromouracil
done
clear
D)
5-methylcytosine
done
clear
View Answer play_arrow
-
question_answer139) The age of the fossil of Dryopithecus on the geological time scale is:
A)
\[75\times {{10}^{6}}\] years back
done
clear
B)
\[25\times {{10}^{6}}\] years back
done
clear
C)
\[2.5\times {{10}^{6}}\] years back
done
clear
D)
\[50\times {{10}^{6}}\] years back
done
clear
View Answer play_arrow
-
question_answer140) A sewage treatment process in which a portion of the decomposer bacteria present in the waste is recycled into the beginning of the process, is called:
A)
cyclic treatment
done
clear
B)
primary treatment
done
clear
C)
activated sludge treatment
done
clear
D)
tertiary treatment
done
clear
View Answer play_arrow
-
question_answer141) Floral features are chiefly used in angiosperms identification because:
A)
flowers are nice to work with
done
clear
B)
flowers are of various colours
done
clear
C)
flowers can be safely pressed
done
clear
D)
reproductive parts are more stable and conservative than vegetative parts
done
clear
View Answer play_arrow
-
question_answer142) Calcitonin is a thyroid hormone which:
A)
elevates potassium level in blood
done
clear
B)
lowers calcium level in blood
done
clear
C)
elevates calcium level in blood
done
clear
D)
has no effect oh calcium
done
clear
View Answer play_arrow
-
question_answer143) A bacterium divides every 35 minutes. If a culture containing \[{{10}^{5}}\] cells per ml is grown for 175 minutes, what will be the cell concentration per ml after 175 minutes?
A)
\[175\times {{10}^{5}}\] cells
done
clear
B)
\[85\times {{10}^{5}}\] cells
done
clear
C)
\[35\times {{10}^{5}}\] cells
done
clear
D)
\[32\times {{10}^{5}}\] cells
done
clear
View Answer play_arrow
-
question_answer144) Mental retardation in man, associated with sex chromosomal abnormality is usually due to:
A)
reduction in X complement
done
clear
B)
increase in X complement
done
clear
C)
moderate increase in Y complement
done
clear
D)
large increase in Y complement
done
clear
View Answer play_arrow
-
question_answer145) Which of the following meristems is responsible for extrastelar secondary growth in dicotyledonous stem?
A)
Phellogen
done
clear
B)
Intra-fascicular cambium
done
clear
C)
Inter-fascicular cambium
done
clear
D)
Inter-calary meristem
done
clear
View Answer play_arrow
-
question_answer146) Lactose is composed of:
A)
glucose + fructose
done
clear
B)
glucose + glucose
done
clear
C)
glucose + galactose
done
clear
D)
fructose + galactose
done
clear
View Answer play_arrow
-
question_answer147) Puccinia forms:
A)
uredia and pycnia on barberry leaves
done
clear
B)
uredia and aecia on wheat leaves
done
clear
C)
uredia and telia on wheat leaves
done
clear
D)
uredia and aecia on barberry leves
done
clear
View Answer play_arrow
-
question_answer148) Radioactive thymidine when added to the medium surrounding living mammalian cells gets incorporated into the newly synthesised DNA. Which of the following types of chromatin is expected to become radioactive if cells arc exposed to radioactive thymidine as soon as they enter the S phase?
A)
Neither heterochromatin nor euchromatin but only the nucleolus
done
clear
B)
Heterochromatin
done
clear
C)
Euchromatin
done
clear
D)
Both (b) and (c)
done
clear
View Answer play_arrow
-
question_answer149) Cellulose, the most important constituent of plant cell wall is made up of:
A)
branched chain of glucose molecules linked by , 1,6 glycosidic bond at the site of branching
done
clear
B)
unbranched chain of glucose molecules linked by , 1,4 glycosidic bond
done
clear
C)
branched chain of glucose molecules linked by , 1, 4 glycosidic bond in straight chain and , 1, 6 glycosidic bond at the site of branching
done
clear
D)
unbranched chain of glucose molecules linked by \[\beta \], 1, 4 glycosidic bond
done
clear
View Answer play_arrow
-
question_answer150) Which of the following pesticides is an acetylcholinesterase inhibitor?
A)
Aldrin
done
clear
B)
Y-BHC
done
clear
C)
Endosulfan
done
clear
D)
Malathion
done
clear
View Answer play_arrow
-
question_answer151) In desert grasslands, which type of animals are relatively more abundant?
A)
Diurnal
done
clear
B)
Arboreal
done
clear
C)
Aquatic
done
clear
D)
Fossorial
done
clear
View Answer play_arrow
-
question_answer152) The long bones are hollow and connected by air passage. They are the characteristics of:
A)
aves
done
clear
B)
mammals
done
clear
C)
reptilia
done
clear
D)
land vertebrates
done
clear
View Answer play_arrow
-
question_answer153) Ulothrix can be described as a:
A)
filamentous alga with flagellated reproductive stages
done
clear
B)
non-motile colonial alga lacking zoospores
done
clear
C)
filamentous alga lacking flagellated reproductive stages
done
clear
D)
membranous alga producing zoospores
done
clear
View Answer play_arrow
-
question_answer154) How many different types of genetically different gametes will be produced by a heterozygous plant having genotype AABbCc?
A)
Two
done
clear
B)
Four
done
clear
C)
Six
done
clear
D)
Nine
done
clear
View Answer play_arrow
-
question_answer155) In vertebrates lacteals are found in:
A)
ileum
done
clear
B)
ischium
done
clear
C)
oesophagous
done
clear
D)
ear
done
clear
View Answer play_arrow
-
question_answer156) Which combination of gases is suitable for fruit ripening?
A)
80% \[{{C}_{2}}{{H}_{4}}\] and 20% \[C{{O}_{2}}\]
done
clear
B)
80% \[C{{O}_{2}}\] and 20% \[C{{H}_{2}}\]
done
clear
C)
80% \[C{{H}_{4}}\] and 20% \[C{{O}_{2}}\]
done
clear
D)
80% \[C{{O}_{2}}\] and 20% \[{{O}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer157) The DNA of E. coli is:
A)
single stranded and linear
done
clear
B)
single stranded and circular
done
clear
C)
double stranded and linear
done
clear
D)
double stranded and circular
done
clear
View Answer play_arrow
-
question_answer158) What is the major cause of diminishing wild life number?
A)
Cannibalism
done
clear
B)
Habitat destruction
done
clear
C)
Felling of trees
done
clear
D)
Paucity of drinking water
done
clear
View Answer play_arrow
-
question_answer159) Biological control compenent is central to advanced agricultural production. Which of the following is used as a third generation pesticide?
A)
Pathogens
done
clear
B)
Pheromones
done
clear
C)
Insect repellants
done
clear
D)
Insect hormone analogues
done
clear
View Answer play_arrow
-
question_answer160) The embryo in sunflower has:
A)
no cotyledon
done
clear
B)
one cotyledon
done
clear
C)
two cotyledons
done
clear
D)
many cotyledons
done
clear
View Answer play_arrow
-
question_answer161) Crossing over in diploid organism is responsible for:
A)
dominance of genes
done
clear
B)
linkage between genes
done
clear
C)
segregation of alleles
done
clear
D)
recombination of linked alleles
done
clear
View Answer play_arrow
-
question_answer162) The main role of bacteria in the carbon cycle involves:
A)
photosynthesis
done
clear
B)
assimilation of nitrogenous compounds
done
clear
C)
chemosynthesis
done
clear
D)
digestion or breakdown of organic compounds
done
clear
View Answer play_arrow
-
question_answer163) A condition of failure of kidney to form urine is called:
A)
deamination
done
clear
B)
entropy
done
clear
C)
anuria
done
clear
D)
none of these
done
clear
View Answer play_arrow
-
question_answer164) Human immuno deficiency virus (HIV) has a protein coat and a genetic material which is:
A)
single stranded DNA
done
clear
B)
single stranded RNA
done
clear
C)
double stranded RNA
done
clear
D)
double stranded PNA
done
clear
View Answer play_arrow
-
question_answer165) Which one of the following is a protein deficiency disease?
A)
Eczema
done
clear
B)
Cirrhosis
done
clear
C)
Kwashiorkor
done
clear
D)
Night blindness
done
clear
View Answer play_arrow
-
question_answer166) Largest sperms in the plants world are found in:
A)
Thuja
done
clear
B)
Pinus
done
clear
C)
Banyan
done
clear
D)
Cycas
done
clear
View Answer play_arrow
-
question_answer167) Recombinant DNA is obtained by cleaving the pro-DNAs by:
A)
primase
done
clear
B)
exonucleases
done
clear
C)
ligase
done
clear
D)
restriction endonuclease
done
clear
View Answer play_arrow
-
question_answer168) The water potential and osmotic potential of pure water are:
A)
100 and zero
done
clear
B)
zero and zero
done
clear
C)
100 and 200
done
clear
D)
zero and 100
done
clear
View Answer play_arrow
-
question_answer169) The chemical knives of DNA are:
A)
ligases
done
clear
B)
polymerases
done
clear
C)
endonucleases
done
clear
D)
transcriptases
done
clear
View Answer play_arrow
-
question_answer170) Which of the following cells, found in testes of rabbit, secrete male hormone?
A)
Leydig's cell
done
clear
B)
Sertoli cells
done
clear
C)
Epithelial cells
done
clear
D)
Spermatocytes
done
clear
View Answer play_arrow
-
question_answer171) What is agent orange?
A)
A biodegradable insecticide
done
clear
B)
A weedicide containing dioxin
done
clear
C)
Colour used in fluorescent lamp
done
clear
D)
A hazardous chemical used in luminous paints
done
clear
View Answer play_arrow
-
question_answer172) Genes that are involved in turning on or off the transcription of a set structural genes are called:
A)
polymorphic genes
done
clear
B)
operator genes
done
clear
C)
redundant genes
done
clear
D)
regulatory genes
done
clear
View Answer play_arrow
-
question_answer173) Farmers have reported over 50% higher yields of rice by using which of the following biofertilizer?
A)
Mycorrhiza
done
clear
B)
Azolla pinnata
done
clear
C)
Cyanobacteria
done
clear
D)
Legume-Rhizobium symbiosis
done
clear
View Answer play_arrow
-
question_answer174) The supersonic jets cause pollution by the thinning of:
A)
\[C{{O}_{2}}\] layer
done
clear
B)
\[S{{O}_{2}}\] layer
done
clear
C)
\[{{O}_{2}}\] layer
done
clear
D)
\[{{O}_{3}}\] layer
done
clear
View Answer play_arrow
-
question_answer175) Albinism is known to be due to an autosomal recessive mutation. The first child of a couple with normal skin pigmentation was an albino. What is the probability that their second child will also be an albino?
A)
100%
done
clear
B)
25%
done
clear
C)
50%
done
clear
D)
75% Since albinism is a recessive character, a child will be albino only if it is homozygous for albinism genes. Since parents have normal skin, it means they are heterozygous. As a result of cross between two heterozygous parents, 25% of the children will be homozygous recessive. The nature of the second child is not affected in any way by the nature of the first child because both are independent events.
done
clear
View Answer play_arrow
-
question_answer176) Transfer of genetic information from one bacterium to another in the transduction process is through:
A)
physical contact between donor and recipient strains
done
clear
B)
conjugation between opposite strain bacterium
done
clear
C)
bacteriophages released from the donor bacterial strain
done
clear
D)
another bacterium having special organ for conjugation
done
clear
View Answer play_arrow
-
question_answer177) The periderm includes:
A)
cork
done
clear
B)
cambium
done
clear
C)
secondary phloem
done
clear
D)
all of these
done
clear
View Answer play_arrow
-
question_answer178) Typhoid fever is caused by:
A)
Giardia
done
clear
B)
Salmonella
done
clear
C)
Shigella
done
clear
D)
Escherichia
done
clear
View Answer play_arrow
-
question_answer179) Most appropriate term to describe the life cycle of Obelia is:
A)
neoteny
done
clear
B)
metagenesis
done
clear
C)
metamorphosis
done
clear
D)
all of these
done
clear
View Answer play_arrow
-
question_answer180) The walking fern is so named because:
A)
its spores are able to walk
done
clear
B)
it is dispersed through the agency of walking animals
done
clear
C)
it propagates vegetatively by its leaf tips
done
clear
D)
it knows how to walk by itself
done
clear
View Answer play_arrow
-
question_answer181) The functional unit of contractile system in striated muscle is:
A)
cross bridge
done
clear
B)
myofibril
done
clear
C)
sarcomere
done
clear
D)
Z-band
done
clear
View Answer play_arrow
-
question_answer182) Bryophytes are dependent on water because:
A)
archegonium has to remain filled with water for fertilization
done
clear
B)
water is essential for fertilization for their homosporous nature
done
clear
C)
water is essential for their vegetative propagation
done
clear
D)
the sperms can easily reach upto egg in the archegonium
done
clear
View Answer play_arrow
-
question_answer183) How many genome types are present in a typical green plant's cell?
A)
Two
done
clear
B)
Three
done
clear
C)
More than five
done
clear
D)
More than ten
done
clear
View Answer play_arrow
-
question_answer184) Restriction endonucleases are:
A)
synthesized by dog
done
clear
B)
present in mammalian cells for degradation of DNA
done
clear
C)
used for in vitro DNA synthesis
done
clear
D)
used in genetic engineering
done
clear
View Answer play_arrow
-
question_answer185) The diversity in the type of beaks of finches adapted to different feeding habits on the Galapagos islands, as observed by Darwin, provides evidence for:
A)
origin of species by natural selection
done
clear
B)
intraspecific variations
done
clear
C)
intraspecific competition
done
clear
D)
interspecific competition
done
clear
View Answer play_arrow
-
question_answer186) DNA elements, which can switch their position, are called:
A)
exons
done
clear
B)
introns
done
clear
C)
cistrons
done
clear
D)
transposons
done
clear
View Answer play_arrow
-
question_answer187) Which one of the following statements about cytochrome \[{{P}_{450}}\] is wrong?
A)
It has an important role in metabolism
done
clear
B)
It contains iron
done
clear
C)
It is a coloured cell
done
clear
D)
It is an enzyme involved in oxidation reactions
done
clear
View Answer play_arrow
-
question_answer188) Genetic drift operates only in:
A)
island populations
done
clear
B)
smaller populations
done
clear
C)
larger populations
done
clear
D)
Mendelian populations
done
clear
View Answer play_arrow
-
question_answer189) An interesting modification of flower shape for insect pollination occurs in some orchids in which a male insect mistakes the pattern on the orchid flower for the female species and tries to copulate with it, thereby pollinating the flower. This phenomenon is called:
A)
mimicry
done
clear
B)
pseudocopulation
done
clear
C)
pseudopollination
done
clear
D)
Pseudoparthenocarpy
done
clear
View Answer play_arrow
-
question_answer190) The response of different organisms to the environmental rhythms of light and darkness is called:
A)
phototaxis
done
clear
B)
phototropism
done
clear
C)
vernalization
done
clear
D)
photoperiodism
done
clear
View Answer play_arrow
-
question_answer191) Warm ocean surge of the peru current recurring every 5 to 8 years or so in the East Pacific of South America is widely known as:
A)
Magnox
done
clear
B)
Gull stream
done
clear
C)
El Nino
done
clear
D)
Aye Aye
done
clear
View Answer play_arrow
-
question_answer192) In the five kingdom system of classification, which single kingdom out of the following can include blue-green algae, nitrogen fixing bacteria and methanogenic archaebacteria ?
A)
Monera
done
clear
B)
Fungi
done
clear
C)
Plantae
done
clear
D)
Protista Monera?It includes all prokaryotic organisms including bacteria and blue-green algae. Protista?It includes eukaryotic unicellular organisms e. g., Amoeba. Plantae?It includes algae, bryophytes, pteridophytes, gymnos perms and argiosperms. Fungi?It includes fungi. Animalia?It includes multicellular animals.
done
clear
View Answer play_arrow
-
question_answer193) Genetic engineering is possible, because:
A)
the phenomenon of transduction in bacteria is well understood
done
clear
B)
we can see DNA by electron microscope
done
clear
C)
we can cut DNA at specific sites by endonucleases like DNAse I
done
clear
D)
restriction endonucleases purified from bacteria can be used in vitro
done
clear
View Answer play_arrow
-
question_answer194) Two bacteria found to be very useful in genetic engineering experiments are:
A)
Nitrosomonas and Klebsiella
done
clear
B)
Escherichia and Agrobacterium
done
clear
C)
Nitrobacter and Azotobacter
done
clear
D)
Rhizobium and Diplococcus
done
clear
View Answer play_arrow
-
question_answer195) Species restricted to a given area are called:
A)
sibling
done
clear
B)
endemic
done
clear
C)
sympatric
done
clear
D)
Allopatric
done
clear
View Answer play_arrow
-
question_answer196) Which one of the following statements is correct?
A)
Homo erectus is the ancestor of man
done
clear
B)
Cro-magnon man's fossil has been found in Ethiopia
done
clear
C)
Australopithecus is the real ancestor of modern man
done
clear
D)
Neanderthal man is the direct ancestor of Homo sapiens
done
clear
View Answer play_arrow
-
question_answer197) In a terrestial ecosystem such as forest, maximum energy is in which trophic level?
A)
\[{{T}_{1}}\]
done
clear
B)
\[{{T}_{2}}\]
done
clear
C)
\[{{T}_{3}}\]
done
clear
D)
\[{{T}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer198) A woman with two genes (one on each 'X' chromosome) for haemophilia and one gene for colour blindness on the 'X? chromosomes marriesa normalman. How will the progeny be?
A)
All sons and daughters haemophilic and colourblind
done
clear
B)
Haemophilic and colourblind daughters
done
clear
C)
50% haemophilic colourblind sons and 50% haemophilic sons
done
clear
D)
50% haemophilic daughters and 50% colourblind daughters
done
clear
View Answer play_arrow
-
question_answer199) Solenocytes are the main excretory structures in:
A)
annelids
done
clear
B)
mollusks
done
clear
C)
echinodermates
done
clear
D)
Platyhelminthes
done
clear
View Answer play_arrow
-
question_answer200) A few organisms are known to grow and multiply at temperatures of \[100-{{105}^{\text{o}}}C\]. They belong to:
A)
thermophilic subaerial fungi
done
clear
B)
marine archaebacteria
done
clear
C)
thermophilic sulphur bacteria.
done
clear
D)
hot spring blue-green algae
done
clear
View Answer play_arrow