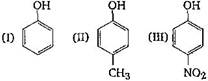
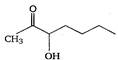
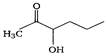
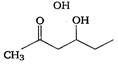
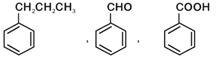
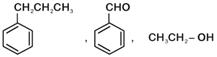
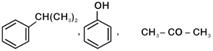
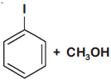
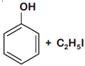
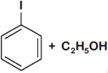
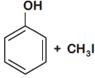
question_answer 1) Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions? [AIPMT 1998]
A)
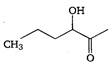
Methyl acetate
done
clear
B)
Acetonitrile
done
clear
C)
Dimethyl ether
done
clear
D)
Acetamide
done
clear
View Answer play_arrow
question_answer 2)
Reaction of
A)
\[RCHOHR\]
done
clear
B)
\[RCHOHC{{H}_{3}}\]
done
clear
C)
\[RC{{H}_{2}}C{{H}_{2}}OH\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 3) The ionization constant of phenol is higher than that of ethanol because: [AIPMT 2000]
A)
phenoxide ion is bulkier than ethoxide
done
clear
B)
phenoxide ion is stronger base than ethoxide
done
clear
C)
phenoxide ion is stabilized through derealization
done
clear
D)
phenoxide ion is less stable than ethoxide
done
clear
View Answer play_arrow
question_answer 4)
The correct acidic order of following is: [AIPMT 2001]
A)
I > II > III
done
clear
B)
III > I > II
done
clear
C)
II > III > I
done
clear
D)
I > III > II
done
clear
View Answer play_arrow
question_answer 5) In preparation of alkene from alcohol using \[A{{I}_{2}}{{O}_{3}}\] which is effective factor? [AIPMT 2001]
A)
Porosity of \[A{{l}_{2}}{{O}_{3}}\]
done
clear
B)
Temperature
done
clear
C)
Concentration
done
clear
D)
Surface area of \[A{{l}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 6) n-propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent: [AIPMT 2002]
A)
\[PC{{l}_{5}}\]
done
clear
B)
reduction
done
clear
C)
oxidation with potassium dichromate
done
clear
D)
ozonolysis
done
clear
View Answer play_arrow
question_answer 7) When phenol is treated with \[CHC{{l}_{3}}\] and \[NaOH\] the product formed is: [AIPMT 2002]
A)
benzaldehyde
done
clear
B)
salicylaldehyde
done
clear
C)
salicylic acid
done
clear
D)
benzoic acid
done
clear
View Answer play_arrow
question_answer 8) The major organic product in the reaction, CH3 O CH(CH3)2 + HI \[\to \] Product is : [AIPMT (S) 2006]
A)
\[C{{H}_{3}}OH+{{(C{{H}_{3}})}_{2}}CHI\]
done
clear
B)
\[IC{{H}_{2}}OCH{{(C{{H}_{3}})}_{2}}\]
done
clear
C)
\[C{{H}_{3}}O\,\,\underset{\begin{smallmatrix} | \\ I \end{smallmatrix}}{\mathop{C}}\,{{(C{{H}_{3}})}_{2}}\]
done
clear
D)
\[C{{H}_{3}}I+{{(C{{H}_{3}})}_{2}}CHOH\]
done
clear
View Answer play_arrow
question_answer 9)
The reaction: [AIPMT (S) 2007] \[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-O-C{{H}_{2}}-C{{H}_{3}}+HI\xrightarrow{Heated}....\] Which of the following compounds will be formed?
A)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-I+C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}+C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}OH+C{{H}_{3}}C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}OH+C{{H}_{3}}-C{{H}_{2}}-I\]
done
clear
View Answer play_arrow
question_answer 10) \[PO_{4}^{3-},\]on heating with periodic acid gives [AIPMT (S) 2009]
A)
\[SO_{4}^{2-}\]
done
clear
B)
2HCOOH
done
clear
C)
\[C{{r}_{2}}O_{7}^{2-}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 11)
Consider the following reaction [AIPMT (S) 2009] \[\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{CHO} \end{smallmatrix}}{\mathop{\text{CHO}}}\,\]\[1.5\times {{10}^{-s}}\]the product Z is
A)
\[4.5\times {{10}^{-10}},\]
done
clear
B)
\[C{{N}^{-}}+C{{H}_{3}}COOH\]
done
clear
C)
\[HCN+C{{H}_{3}}CO{{O}^{-}}\]
done
clear
D)
\[3.0\times {{10}^{5}}\]
done
clear
View Answer play_arrow
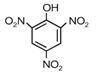
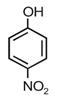
question_answer 12) Given are cyclohexanol (I), acetic acid (II), 2, 4, 6-trinitrophenol (III) and phenol (IV). In these, the order of decreasing acidic character will be [AIPMT (S) 2010]
A)
III > II > IV > I
done
clear
B)
II > III > I >IV
done
clear
C)
II > III > IV > I
done
clear
D)
III > IV > II > I
done
clear
View Answer play_arrow
question_answer 13) When glycerol is treated with excess of HI, it produces [AIPMT (M) 2010]
A)
2-iodopro'pane
done
clear
B)
allyl iodide
done
clear
C)
propene
done
clear
D)
glycerol triiodide
done
clear
View Answer play_arrow
question_answer 14) Which one of the following compounds will be most readily dehydrated? [AIPMT (M) 2010]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 15)
In the following reactions, [AIPMT (S) 2011] (I) \[\text{C}{{\text{H}}_{\text{3}}}-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{3}} & \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{CH}}}\,-\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{OH} \end{smallmatrix}}{\mathop{\text{CH}}}\,-\text{C}{{\text{H}}_{\text{3}}}\xrightarrow[{}]{{{\text{H}}^{\text{+}}}\text{/Heat}}\] \[{{\left( \overset{\text{A}}{\mathop{\begin{align} & \text{major} \\ & \text{product} \\ \end{align}}}\, \right)}^{\text{+}}}\left( \overset{\text{B}}{\mathop{\begin{align} & \text{major} \\ & \text{product} \\ \end{align}}}\, \right)\] (ii)\[\text{A}\xrightarrow[\text{in}\,\text{absence}\,\text{of}\,\text{peroxide}]{\text{HBr,dark}}\]\[{{\left( \overset{\text{C}}{\mathop{\begin{align} & \text{major} \\ & \text{product} \\ \end{align}}}\, \right)}^{\text{+}}}\left( \overset{\text{D}}{\mathop{\begin{align} & \text{major} \\ & \text{product} \\ \end{align}}}\, \right)\] the major products and are respectively
A)
\[\text{C}{{\text{H}}_{\text{3}}}-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,\text{=}\,\text{CH}-\text{C}{{\text{H}}_{\text{3}}}\]and \[\text{C}{{\text{H}}_{\text{3}}}-\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{Br} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,-\text{CH}{{ & }_{\text{2}}}-\text{C}{{\text{H}}_{\text{3}}}\]
done
clear
B)
\[\text{C}{{\text{H}}_{\text{3}}}-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,\text{=}\,\text{CH}-\text{C}{{\text{H}}_{\text{3}}}\]and \[\text{C}{{\text{H}}_{\text{3}}}-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,-\underset{\begin{smallmatrix} | \\ \text{Br} \end{smallmatrix}}{\mathop{\text{CH}}}\,-\text{C}{{\text{H}}_{\text{3}}}\]
done
clear
C)
\[\text{C}{{\text{H}}_{2}}=\,\,\,\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,-\text{C}{{\text{H}}_{2}}-\text{C}{{\text{H}}_{\text{3}}}\]and \[\text{C}{{\text{H}}_{\text{3}}}-\underset{\begin{smallmatrix} \text{ }\!\!|\!\!\text{ } \\ \text{Br} \end{smallmatrix}}{\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}}\,-\text{C}{{\text{H}}_{\text{2}}}-\text{C}{{\text{H}}_{\text{3}}}\]
done
clear
D)
\[\text{C}{{\text{H}}_{\text{2}}}\text{=}\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\text{C}}}\,-\text{C}{{\text{H}}_{\text{2}}}-\text{C}{{\text{H}}_{\text{3}}}\]and \[\underset{\begin{smallmatrix} | \\ \text{Br} \end{smallmatrix}}{\mathop{\text{C}{{\text{H}}_{\text{2}}}}}\,-\overset{\begin{smallmatrix} \text{C}{{\text{H}}_{\text{3}}} \\ \text{ }\!\!|\!\!\text{ } \end{smallmatrix}}{\mathop{\,\text{CH}\,}}\,-\text{C}{{\text{H}}_{\text{2}}}-\text{C}{{\text{H}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 16) Which of the following compounds can be used as antifreeze in automobile radiators? [AIPMT (M) 2012]
A)
Methyl alcohol
done
clear
B)
Glycol
done
clear
C)
Nitrophenol
done
clear
D)
Ethyl alcohol
done
clear
View Answer play_arrow
question_answer 17) Among the following sets of reactants which one produces anisole? [AIPMT 2014]
A)
\[C{{H}_{3}}CHO;RMgX\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}OH;NaOH;C{{H}_{3}}l\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}OH;\]neutral \[FeC{{l}_{3}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}-C{{H}_{3}};C{{H}_{3}}COCl;AlC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 18) Which of the following will not be soluble in sodium hydrogen carbonate? [AIPMT 2014]
A)
2, 4, 6-trinitrophenol
done
clear
B)
Benzoic acid
done
clear
C)
o-nitrophenol
done
clear
D)
Benzenesulphonic acid
done
clear
View Answer play_arrow
question_answer 19)
Which of the following reaction(s) can be used for the preparation of alkyl halides? [NEET 2015 (Re)] I. \[C{{H}_{3}}C{{H}_{2}}OH+HCl\xrightarrow{anh.ZnC{{l}_{2}}}\] II. \[C{{H}_{3}}C{{H}_{2}}OH+HCl\xrightarrow{\,}\] III. \[{{(C{{H}_{3}})}_{3}}COH+HCl\xrightarrow{\,}\] IV. \[{{(C{{H}_{3}})}_{2}}CHOH+HCl\xrightarrow{\text{anh}\text{.}\,\text{ZnC}{{\text{l}}_{\text{2}}}}\]
A)
I, III and IV
done
clear
B)
I and II
done
clear
C)
Only IV
done
clear
D)
III and IV
done
clear
View Answer play_arrow
question_answer 20) Which copper is heated with conc. \[\text{HN}{{\text{O}}_{\text{3}}}\]it produces [NEET - 2016]
A)
\[\text{Cu}{{\left( \text{N}{{\text{O}}_{\text{3}}} \right)}_{\text{2}}}\]and \[\text{N}{{\text{O}}_{\text{2}}}\]
done
clear
B)
\[\text{Cu}{{\left( \text{N}{{\text{O}}_{\text{3}}} \right)}_{\text{2}}}\] and NO
done
clear
C)
\[\text{Cu}{{\left( \text{N}{{\text{O}}_{\text{3}}} \right)}_{\text{2}}},\]NO and \[\text{N}{{\text{O}}_{\text{2}}}\]
done
clear
D)
\[\text{Cu}{{\left( \text{N}{{\text{O}}_{\text{3}}} \right)}_{\text{2}}}\]and \[\text{N}{{\text{O}}_{\text{2}}}\]
done
clear
View Answer play_arrow
question_answer 21)
The reaction Can be classified as:- [NEET - 2016]
A)
Williamson ether synthesis reaction
done
clear
B)
Alcohol formation reaction
done
clear
C)
Dehydration reaction
done
clear
D)
Williamson alcohol synthesis reaction
done
clear
View Answer play_arrow
question_answer 22) The heating of phenyl-methyl ethers with HI produces. [NEET-2017]
A)
Benzene
done
clear
B)
Ethyl chlorides
done
clear
C)
Iodobenzene
done
clear
D)
Phenol
done
clear
View Answer play_arrow
question_answer 23) Which one is the most acidic compound? [NEET-2017]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
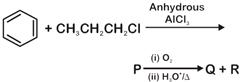
question_answer 24)
Identify the major products P, Q and R in the following sequence of reactions: [NEET - 2018]
A)
P Q R
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 25) The compound A on treatment with Na gives B, and with \[\text{PC}{{\text{l}}_{\text{5}}}\] gives C. B and C react together to give diethyl ether. A, B and C are in the order [NEET - 2018]
A)
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{Cl, }{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\text{,}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{OH}\]
done
clear
B)
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{OH, }{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{Cl,}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{ONa}\]
done
clear
C)
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{OH,}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\text{,}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{Cl}\]
done
clear
D)
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{OH, }{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{ONa, }{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{Cl}\]
done
clear
View Answer play_arrow
question_answer 26) Anisole on cleavage with HI gives [NEET 2020]
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow